What Is The Ground
The ground state electron configuration of ground state gaseous neutral iron is . 3d6. 4s2 and the term symbol is 5D4.
What is the ground state electron configuration for Fe2 +?, The electron configuration for Fe2+ will be 1s2 2s2 2p6 3s2 3p6 4s2 3d4 because it has lost two electrons.
Furthermore, What is the ground state of electron?, The ground state of an electron, the energy level it normally occupies, is the state of lowest energy for that electron. There is also a maximum energy that each electron can have and still be part of its atom.
Finally, What is the ground state of an element?, The ground state is the state that is occupied by the most part of the atoms of the same element at room temperature, because it is lower in energy.
Definition Of Ground State
The ground state of an atomic nucleus, atom, or molecule is its lowest energy state.Higher energy states are described as excited states.
The ground state applies to any quantized property of a particle.In chemistry,
electron ground states
rotational ground states
of atoms and molecules are important, as are their excited states.
At room temperature, most molecules are in electron and vibrational ground states higher temperatures are needed for molecules to enter excited states.However, most molecules are in an excited rotational state at room temperature, because less energy is needed for a molecule to enter a rotational excited state than an electron or vibrational excited state.
Which One Is In The Ground State
The ground state of a quantum-mechanical system is its lowest-energy state the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In quantum field theory, the ground state is usually called the vacuum state or the vacuum.
Read Also: Paris Jackson Parents
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
How Much Energy Does It Take To Shoot An Arrow
Archery as a sport or a means of defense has existed for centuries. At rest, there is no tension on the bowstring and no force on the arrow. When the string and arrow are pulled back, we now have a situation where kinetic energy has been converted to potential energy .
The archer releases the arrow and the potential energy is translated into kinetic energy as the arrow moves. It turns out that electrons behave the same way when energy is put into the system or released from the system.
The electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible. The ground state of an atom is the lowest energy state of the atom. When those atoms are given energy, the electrons absorb the energy and move to a higher energy level. These energy levels of the electrons in atoms are quantized, meaning again that the electron must move from one energy level to another in discrete steps rather than continuously. An excited state of an atom is a state where its potential energy is higher than the ground state. An atom in the excited state is not stable. When it returns back to the ground state, it releases the energy that it had previously gained in the form of electromagnetic radiation.
Neon signs are familiar examples of gas discharge tubes. However, only signs that glow with the red-orange color seen in the figure are actually filled with neon. Signs of other colors contain different gases or mixtures of gases.
Recommended Reading: Geometry Dash 1 20
Returning To The Ground State
Particles in excited states can return to the ground state by releasing energy.The energy is lost in the form of electromagnetic radiation.
- When electrons in atoms or molecules emit energy and fall into lower energy sublevels, including into the ground state, the energy takes the form of visible or ultraviolet light.
- Vibrational energy is lost by the emission of infrared light.
- Rotational energy is lost by the emission of microwave or far infrared radiation.
How Do You Know If An Atom Is In The Ground State
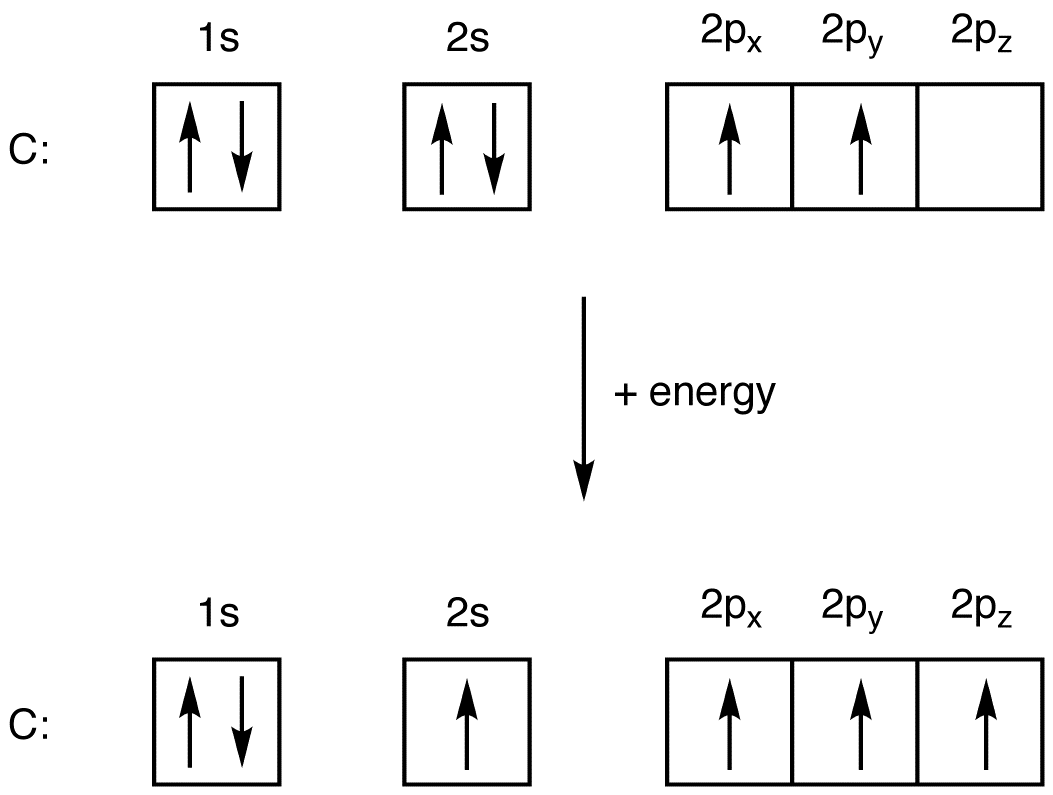
A ground-state atom is an atom in which the total energy of the electrons can not be lowered by transferring one or more electrons to different orbitals. That is, in a ground-state atom, all electrons are in the lowest possible energy levels. eg: Consider a carbon atom whose electron configuration is the following.
You May Like: G Unit Physics
What Is Excited State
Excited state of an atom refers to the state which has a higher energy than the ground state of that atom. Here, one or more electrons are not at their lowest possible energy level. Electrons have moved to a higher energy level by absorbing energy provided from outside. But, in order to move to an excited state, the provided amount of energy should be equal to the energy difference between the two energy levels. Otherwise, no excitation would take place.
However, excited state is not stable since higher energy levels are not stable and atoms tend to return to the ground state by emitting the absorbed energy. This emission leads to the formation of an electromagnetic spectrum that has emission lines.
Figure 2: Emission of Absorbed Energy from an Excited State
The lifetime of an excited state is very short since the excited state is unstable due to its high energy. Here, the distance between the atomic nucleus and the electrons are not the least possible distances.
How Does An Atom In The Ground State Become Excited
Atoms become excited when electrons go from the ground state to an orbital level requiring more energy to exist there. This happens through some energy absorbtion whether that be heat, electricity or light.
ground state. The state of a physical system having the lowest possible potential energy. For example, an electron in the lowest energy orbital in a hydrogen atom is in a ground state.
Recommended Reading: Olaplex Vs Redken
What Is Ground State
Ground state refers to the state in which all electrons in a system are in the lowest possible energy levels. Therefore, the ground state is known to have no energy when compared to an excited state because the electrons are in a zero energy level. The ground state is also called the vacuum state.
When energy is provided to an atom in the ground state, it can move to an excited state by absorbing energy. But the lifetime of the excited state is less, Thus, the atom returns to the ground state, emitting the absorbed energy as shown in the following image.
Figure 1: Emission of Absorbed Energy
Therefore, ground state is highly stable when compared to the excited state and has a longer lifetime. In ground state atoms, the distance between electrons and the atomic nucleus has the least possible distance. The electrons reside closer to the atomic nucleus.
Ground State Electron Configurations
Electron configurations are written so as to clearly show the number of electrons in the atom as well as the number of electrons in each orbital. Each orbital is written in sequence, with the number of electrons in each orbital written in superscript to the right of the orbital name. The final electron configuration is a single string of orbital names and superscripts. For example, sodium has 11 protons and 11 electrons. Its electron configuration is 1s2 2s2 2p6 3s1. If you add up the superscripts, you can see that it adds up to the 11 electrons characteristic of sodium. To determine the order of notation for electron configurations, the orbitals with the lowest energy level will be filled in first, shown by the order noted in the diagram below.
Also Check: Algebra 1 Eoc 2015
What Should I Look For In A 5s Audit
Success in using the 5S audit forms For more reliable and meaningful results while using the 5S audit form, the test must score multiple dimensions and include the system configuration, know-how levels, observed cleanliness results, organizational system approach, as well as the frequency of tagging.
What Does Ground State Mean Chemistry

3.9/5DefinitionGround Stateground stateisstatestatesexcited states
In this regard, what does ground state mean?
A ground–state atom is an atom in which the total energy of the electrons can not be lowered by transferring one or more electrons to different orbitals. That is, in a ground–state atom, all electrons are in the lowest possible energy levels. eg: Consider a carbon atom whose electron configuration is the following.
Secondly, what is ground and excited state? The ground state describes the lowest possible energy that an atom can have. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. An electron is normally in its ground state, the lowest energy state available.
Similarly, it is asked, what is an example of ground state?
The ground state is the state that is occupied by the most part of the atoms of the same element at room temperature, because it is lower in energy. For example, a simple six electron atom as the carbon atom has the most stable electronic configuration represented by 1s² 2s² 2p².
Why is ground state important chemistry?
The ground state refers to an unexcited atom where the electrons are in their lowest energy levels. Using the ground state of electrons can also tell us the fill order of electrons in an atom. According to the aufbau principle, electrons fill the lowest available energy level first.
Don’t Miss: Geometry Basics Segment Addition Postulate Worksheet Answer Key
What Is Ground State And Excited State Of An Atom
The ground state describes the lowest possible energy that an atom can have. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state.
Also to know is, what is the excited state of an atom?
An excited–state atom is an atom in which the total energy of the electrons can be lowered by transferring one or more electrons to different orbitals. That is, in an excited–state atom not all electrons are in the lowest possible energy levels. eg. Consider a carbon atom whose electron configuration is the following.
Also, what happens when electrons move from the excited state to the ground state? When an electron absorbs energy, it jumps to a higher orbital. An electron in an excited state can release energy and ‘fall’ to a lower state. When the electron returns to the ground state, it can no longer release energy, but can absorb quanta of energy and move up to excitation states .
Subsequently, question is, what is the difference between an excited and ground state of an atom?
The main difference between ground state and excited state is that ground state is a state whereas electrons in a system are in the lowest possible energy levels whereas excited state is any state of the system that has a higher energy than the ground state.
What is Hund rule?
You May Like Also
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Read Also: Lewis Dot Ccl4
Why Is Electron Configurations Important
The Important Aspect Is That We Realize That Knowing Electron Configurations Helps Us Determine The Valence Electrons On An Atom. This Is Important Because Valence Electrons Contribute To The Unique Chemistry Of Each Atom. This Is Important When Describing An Electron Configuration In Terms Of The Orbital Diagrams.
What Is The Difference Between An Atom’s Ground State And Excited State
4.8/5What is the difference betweenground stateexcited stateatomground statestateatomatomstateexcited stateatomstatestateof the answer
The ground state describes the lowest possible energy that an atom can have. Atoms may occupy different energy states. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state.
One may also ask, what is an excited state of an atom? An excited–state atom is an atom in which the total energy of the electrons can be lowered by transferring one or more electrons to different orbitals. That is, in an excited–state atom not all electrons are in the lowest possible energy levels. eg. Consider a carbon atom whose electron configuration is the following.
Also, what is the difference between the ground state and the excited state of electron positions?
The main difference between ground state and excited state is that ground state is a state whereas electrons in a system are in the lowest possible energy levels whereas excited state is any state of the system that has a higher energy than the ground state.
What happens when electrons move from the excited state to the ground state?
Also Check: Domain And Range Worksheet 2 Answer Key Algebra 1
Ground State Excited States
An atom can be at its lowest energy state, ground state, or gain energy to move to excited states, higher energy levels, in discrete steps.
The electrons in an atom tend to be arranged in such a way that the energy of the atom is as low as possible, also known as the ground state.
When those atoms are given energy, the electrons absorb the energy and move to a higher energy level. These energy levels of the electrons in atoms are quantized, meaning again that the electron must move from one energy level to another in discrete steps rather than continuously. An excited state of an atom is a state where its potential energy is higher than the ground state.
A is a source of energy that electrons can absorb to make a quantum jump to a higher energy level: when the energy of the photon is equal to the energy difference between the discrete steps.
An atom in the excited state is not stable. When it returns to the ground state, it releases the energy that it had previously gained in the form of electromagnetic radiation. The electron can reach its ground state level either with one direct jump or with shorter jumps via intermediate levels. If an excited electron emits a photon, the energy hf of that photon equals the energy difference between the initial energy level of the electron and a lower level. By measuring the photons emitted the size of these energy levels can be determined.
Apa Yang Dimaksud Dengan Keadaan Dasar Kimia
3.9/5DefinisiKeadaan DasardasaradalahkeadaanKeadaankeadaan tereksitasi.
Atom keadaandasar adalah atom yang energi total elektronnya tidak dapat diturunkan dengan mentransfer satu atau lebih elektron ke orbital yang berbeda. Artinya, dalam atom keadaandasar , semua elektron berada dalam tingkat energi serendah mungkin. misalnya: Pertimbangkan atom karbon yang konfigurasi elektronnya adalah sebagai berikut.
Juga, apa itu keadaan dasar dan keadaan tereksitasi? Keadaan dasar menggambarkan energi serendah mungkin yang dapat dimiliki atom. Keadaan tereksitasi adalah tingkat energi atom, ion, atau molekul di mana elektron berada pada tingkat energi yang lebih tinggi daripada keadaan dasarnya . Sebuah elektron biasanya dalam keadaan dasar , keadaan energi terendah yang tersedia.
Di sini, apa contoh keadaan dasar?
Keadaan dasar adalah keadaan yang ditempati oleh sebagian besar atom unsur yang sama pada suhu kamar, karena energinya lebih rendah. Misalnya , atom enam elektron sederhana sebagai atom karbon memiliki konfigurasi elektron paling stabil yang diwakili oleh 1s² 2s² 2p².
Mengapa keadaan dasar kimia penting?
Keadaan dasar mengacu pada atom yang tidak tereksitasi di mana elektron berada pada tingkat energi terendah. Menggunakan keadaan dasar elektron juga dapat memberi tahu kita urutan pengisian elektron dalam sebuah atom. Menurut prinsip aufbau, elektron mengisi tingkat energi terendah yang tersedia terlebih dahulu.
Don’t Miss: Holt Geometry Chapter 2 Test
What Are The 5 Steps Of 5s
The five words in 5S represent the five steps to accomplish this goal. They are sort, set, shine, standardize and sustain. Lean bases the words on the original Japanese: seiri, seiton, seiso, seiketsu and shitsuke. 5S is a key component in eliminating the eight wastes of Lean when setting up a workstation.
Examples Of Ground State In A Sentence
ground state Ars Technicaground state The Conversationground stateScientific Americanground state Quanta Magazineground stateForbesground stateScientific Americanground state Los Angeles Timesground state Wired
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘ground state.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
You May Like: Physics Finding Acceleration