Steepness Of The Concentration Gradient
Since diffusion is powered primarily by the probability of molecules moving away from a region of higher saturation, it immediately follows that when the medium has a very low concentration of the solute, the probability of a molecule diffusing away from the central area is higher. For instance, in the example about the diffusion of iodine gas, if the crucible is placed in another closed container and iodine crystals are heated for an extended period of time, the rate at which the purple gas seems to âdisappearâ at the mouth of the crucible will reduce. This apparent slowing down is due to the fact that, over time, the larger container begins to have enough iodine gas that some of it will be moving âbackwardsâ towards the crucible. Even though this is random non-directed movement, with a large bulk, it can create a scenario where there is no net movement of gas from the container.
What Is A Facilitated Diffusion Example In Real Life
There are plenty of examples of facilitated diffusion in the real world and for fact facilitated diffusion occurs probably every second in your body, it is just that you cannot notice them. There are plenty of tiny cells present within the body that function your body by generating energy. This energy can only be produced when the cells intake certain substances, but if any other type of substance is let inside it could damage the cell.
Facilitated diffusion takes care of this situation where a certain substance can diffuse to any concentration gradient. Also, a certain type of protein called transmembrane greatly assists the cells in the intake and outtake of the substances.
What is facilitated diffusion in our body if you ask then the oxygens affinity towards red blood cells and the absorption of glucose molecules into cells are examples of facilitated diffusion in our bodies? We can also conclude that almost every living and non-living thing adapt facilitated diffusion when we define facilitated diffusion in biology.
Examples Of Facilitated Diffusion
D. All of the above
D
3. Which of these statements is NOT true?A. Glucose undergoes facilitated diffusion through a transmembrane channelB. Water can move across a membrane even in the absence of aquaporinsC. The potassium ion transporter has a thousand-fold greater affinity for potassium ions over sodium ionsD. All of the above
A
Cite This Article
Biology Dictionary
You May Like: Practice Hall Gold Geometry
State The Significance Of Diffusion
Diffusion is a very important process occurring in all living beings. All living organisms exhibit one or the other form of diffusion, allowing the movement of the molecules during various metabolic or cellular processes.
Learn more about diffusion, its definition, types examples, and other related topics at
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Need For Facilitated Diffusion
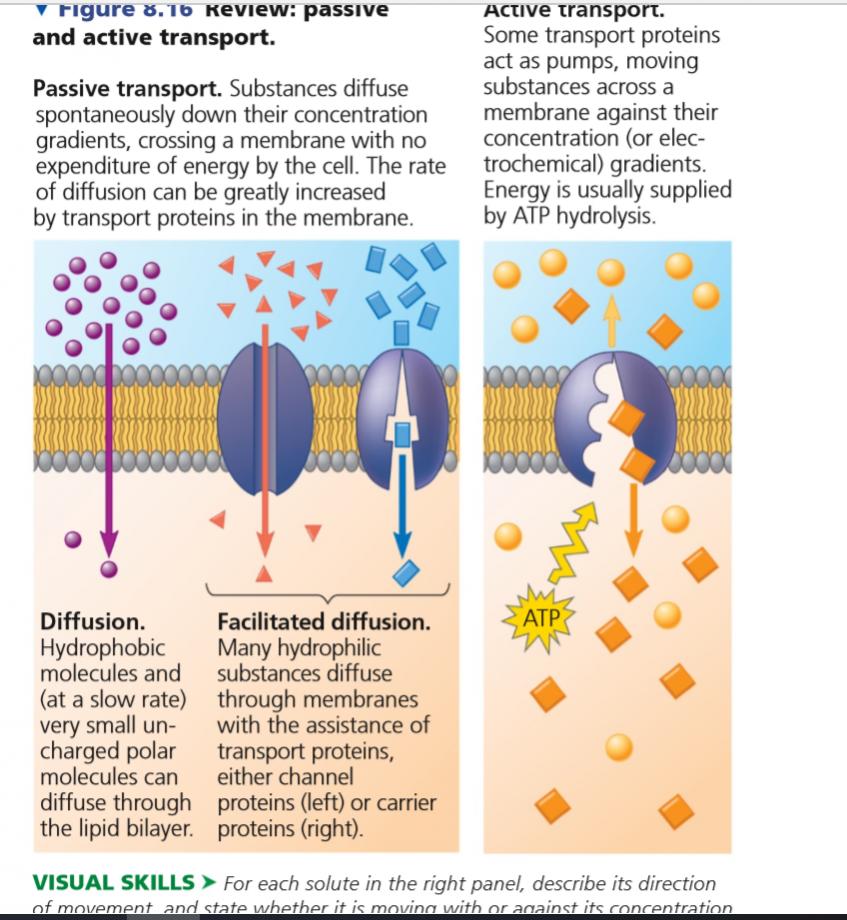
Cell membranes are only freely permeable to a very limited class of molecules. They must be small in size, and non-polar. While this allows molecules like water, oxygen and carbon dioxide to diffuse across membranes, it precludes practically every biopolymer, most nutrients and many important small molecules.
For instance, glucose is a relatively large molecule that cannot diffuse directly through the lipid bilayer. Similarly, important ions like sodium, potassium or calcium ions are charged and therefore repelled by the lipophilic core of cell membranes. Amino acids and nucleic acids are polar, often charged and too large to use simple diffusion to enter and exit cells. Occasionally, even the bulk movement of water across membranes cannot occur quickly through the lipid bilayer.
In these situations, facilitated diffusion, through integral membrane proteins, becomes important. These transmembrane proteins are usually of two types those that act like carriers and those that form channels across the membrane.
Read Also: The Founder Of Behaviorism
What Causes Diffusion And What Happens During The Process
The random movement of molecules existing in any state of solid, liquid, or gas increases the kinetic energy of the system. Since diffusion equalizes the concentration of the substance on both sides of the region, it helps the solution to attain the state of equilibrium or minimum randomness through this process.
If Ficks laws can describe a diffusion process, it is called a normal or Fickian diffusion, otherwise, it is named as anomalous or non-Fickian diffusion.
Diffusion Of Electrons In Solids
When the density of electrons in solids is not in equilibrium, diffusion of electrons occurs. For example, when a bias is applied to two ends of a chunk of semiconductor, or a light shines on one end , electrons diffuse from high density regions to low density regions , forming a gradient of electron density. This process generates current, referred to as diffusion current.
Diffusion current can also be described by Fick’s first law
- J ,
where J is the diffusion current density per unit area per unit time, n is the electron density, x is the position .
Also Check: Prentice Hall Geometry Activities Games And Puzzles Answers
What Factors Affect Diffusion
The different factors that affect diffusion either individually or collectively are:
1) Temperature: Warmer the temperature, higher is the rate of diffusion.
2) Area of Interaction: More the surface area of interacting molecules, higher is the rate of diffusion.
3) The Extent of the Concentration Gradient: Greater the difference in concentration between the regions, higher is the rate of diffusion.
4) Diffusion Distance: Smaller the distance covered by the diffusing molecules, faster is the rate of diffusion.
5) Types of Diffusing Materials: At a particular temperature, materials with lighter atoms diffuse faster than heavier ones.
6) Particle Size: At any given temperature, the diffusion of a smaller particle will be more rapid than the larger ones.
What Assists The Movement Of Substances By Facilitated Diffusion In A System
What is facilitated diffusion affected by that can either slow down or quicken up the process. The factors affecting facilitated diffusion are:
-
Temperature: Usually when the surrounding temperature of a cell is higher, the movement of the substance through the transmembrane proteins is faster. This is due to the greater energy levels exhibited.
-
Size: When it comes to cells, the intake substance varies in size. The larger sized particles will have a harder time getting through the transmembrane proteins than its smaller counterparts.
-
Concentration Amounts: The description of facilitated diffusion states the movement of particles from a higher concentration area to a low concentration area. Therefore, based on the concentration levels, the movement speed will vary.
-
The Number of Transmembrane Proteins: For a facilitated diffusion to take place there must be these so-called transmembrane proteins present according to what is facilitated diffusion is defined as. So, if there are many sites present the movement will also be greater and vice-versa.
Recommended Reading: Renate Blauel 2018
Cell Membranes Problem Set
Crossing a membrane by simple diffusion can be distinguished from facilitated diffusion because:
A. Simple diffusion does not require energy: facilitated diffusion requires a source of ATP. B. Simple diffusion can only move material in the direction of a concentration gradient facilitated diffusion moves materials with and against a concentration gradient. C.
Simple diffusion is not saturable facilitated diffusion rates are limited by the number of functional membrane proteins and can be saturated
Diffusion means that the net movement of particles is from an area of high concentration to low concentration. If the particles can move through the lipid bilayer by simple diffusion, then nothing limits the number that can fit through the membrane. Thus, the rate of diffusion increases linearly as we add more particles to one side of the membrane.
If the particles can only pass through protein channels, then the rate of diffusion is determined by the number of channels as well as the number of particles. Once the channels operate at their maximal rate, a further increase in particle numbers no longer increases the apparent rate of diffusion. At this limited rate we describe the protein channel as being saturated.
D.
The Mass Transport Regime
Diffusion to a microelectrode is notably different than that to a conventional electrode, including mm-size electrodes. For a simple fast electron transfer process, the application of a potential step from a value where no current flows to one where the reaction is diffusion controlled produces different diffusion regimes. At short times, the diffusion layer is very thin relative to the electrode. Diffusion to and from the electrode is planar and the microelectrode behaves like a conventional electrode. As time increases, the diffusion layer thickness becomes comparable, and then larger than the dimensions of the electrode. The diffusion regime evolves from planar to spherical and yields a steady-state rate of mass transport to the electrode. So in contrast to the constant planar diffusion regime observed with large electrodes, a microelectrode shifts from planar to spherical diffusion. This, of course, depends on the characteristic dimension of the electrode. Below 50 µm, the microelectrode can fully develop its diffusion layer without influence from natural convection. Larger electrodes produce thicker diffusion layers which soon become affected by natural convection. In this case, the steady rate of mass transport observed is greater than that for diffusion alone.
G. Pritchard, in, 2000
Don’t Miss: 5.8 Special Right Triangles Worksheet
Osmosis And Semipermeable Membranes
Osmosis is the movement of water through a semipermeable membrane according to the concentration gradient of water across the membrane, which is inversely proportional to the concentration of solutes. Semipermeable membranes, also termed selectively permeable membranes or partially permeable membranes, allow certain molecules or ions to pass through by diffusion.
While diffusion transports materials across membranes and within cells, osmosis transports only water across a membrane. The semipermeable membrane limits the diffusion of solutes in the water. Not surprisingly, the aquaporin proteins that facilitate water movement play a large role in osmosis, most prominently in red blood cells and the membranes of kidney tubules.
Pros And Cons Of Diffusion
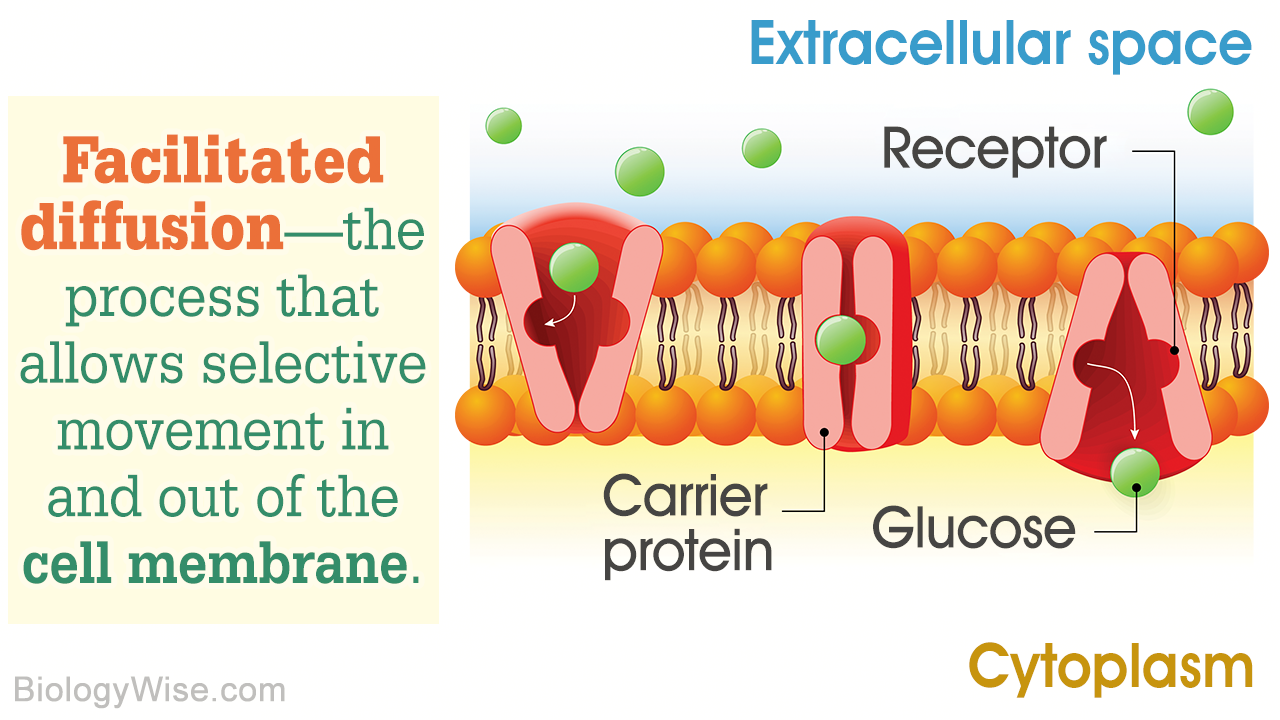
On the plus side, the diffusion process is “free” compared to other forms of transport in that it does not require energy. This is a major asset given that efficiency is enormously desirable in biological systems and energy, just as in the “macro” world, is at a premium.
The down side of diffusion is that is it obviously insufficient to move substances up a concentration gradient, and it is not difficult to envision a scenario in which molecules are needed inside a cell despite an already higher concentration of these substances on the inside than on the outside. More often, such substances must be moved across an electrochemical gradient.
This is a different physical form of resistance, but it’s one that only an investment of ATP can overcome. This is done using membrane “pumps” that continually fight the tide of the electrochemical gradient that opposes their work.
Related Articles
Also Check: Segment Addition Postulate Algebra Calculator
What Is Diffusion In Biology
Diffusion is a type of transport that moves molecules or compounds in or out of a cell. There are three main types of diffusion, which include simple diffusion, channel diffusion and facilitated diffusion.
Simple diffusion occurs when small, nonpolar molecules transport through the selectively permeable cell membrane. Molecules that are able to pass through the membrane must be hydrophobic so that they can move through the hydrophobic region of the lipid bilayer region. Simple diffusion is a passive process that does not require energy or a membrane protein.
Channel diffusion is also a type of passive transport that occurs with the help of membrane transport proteins. These proteins are embedded in the cell membrane and can open and close to allow molecules or compounds into or out of the cell. Channel diffusion is easily regulated by the membrane proteins. In general, ions and charged particles are the types of molecules that utilize channel diffusion.
The final type of diffusion is facilitated diffusion. This type of diffusion also utilizes protein carriers that are embedded into the cells membrane. These protein carriers bind to compounds, then change their shape. Next, they release the compound into or out of the cell and regain their shape. Facilitated diffusion is also a passive process.
Corrosionpedia Explains Diffusion Coefficient
Diffusion coefficients are among the variables that induce corrosion. With this, accurate computation of diffusivity must be performed to prevent corrosion.
Measuring the diffusion coefficient is vital in predicting corrosion in concrete substances. This is used in industries such as maritime, and in structures such as highways, bridge decks and other concrete.
The diffusion coefficient is the physical constant that depends on properties like the size of molecules of the substance undergoing diffusion. Apart from molecular size, other factors that affect diffusivity are pressure and temperature. The diffusivity of one particular substance to another is typically identified through experimenting.
Diffusion coefficients can be computed in liquids, gases and multiple media that usually involve factors such as changes in temperature and pressure. Close monitoring is important since diffusivity in liquid and gaseous phases can vary, which means that diffusion involves molecular movement through molecule layers of similar or different substances.
This only implies that the diffusion coefficient of various substances differs depending on the phases and factors involved. Proper levels should be maintained all the time in order to mitigate or prevent the damaging effects of corrosion.
You May Like: Which Of The Following Perspectives Dominated American Psychology For Decades
Factors That Affect Diffusion
Molecules move constantly in a random manner at a rate that depends on their mass, their environment, and the amount of thermal energy they possess, which in turn is a function of temperature. This movement accounts for the diffusion of molecules through whatever medium in which they are localized. A substance will tend to move into any space available to it until it is evenly distributed throughout it. After a substance has diffused completely through a space removing its concentration gradient, molecules will still move around in the space, but there will be no net movement of the number of molecules from one area to another. This lack of a concentration gradient in which there is no net movement of a substance is known as dynamic equilibrium. While diffusion will go forward in the presence of a concentration gradient of a substance, several factors affect the rate of diffusion:
The Effective Friction Coefficient
In chapter 2 on diffusion of non-interacting Brownian particles, we have seen that the diffusion coefficient D0 is related through the Einstein relation D0 = kBT/ with the friction coefficient of the Brownian particle with the solvent. The mean-squared displacement of a Brownian particle is thus related to the stationary velocity that the particle attains when subjected to an external force. Now suppose that the Brownian particle interacts with neighbouring Brownian particles. The pure solvent is thus replaced by a dispersion, and the friction coefficient is now an effective friction coefficient eff, the numerical value of which is affected by the interactions of the tracer particle with the host particles. It is tempting toassume an Einstein relation between the long-time self diffusion coefficient and the effective friction coefficient, that is,
The problem is thus to calculate the stationary average velocity < vt> of the tracer particle due to an external force Fext. The brackets < > denote ensemble averaging over fluctuations of the actual velocity due to interactions with the host Brownian particles. We have seen in chapter 5 that the velocity of the tracer particle is related linearly to the hydrodynamic forces F
Guy Denuault, … Kirsty-Jo Williams, in, 2007
Don’t Miss: Qualitative Data Definition Ap Human Geography
Factors Affecting Facilitated Diffusion
Brownian motion is the force behind the diffusion of fluids. The main factors affecting the process of facilitated diffusion are:
-
Temperature- As the temperature increases, the movement of the molecules increases due to an increase in energy.
-
Concentration- The movement of the molecules takes place from the region of higher concentration to lower concentration.
-
Diffusion Distance- The diffusion rate is faster through smaller distance than through the larger distance. For eg., gas diffuses much faster through a thin wall than through a thick wall.
-
Size of the molecules- The smaller molecules are lighter and hence diffuse faster than the larger molecules.
Facilitated Diffusion Across Membranes
Diffusion is ubiquitous across the biosphere. It is seen in the movement of air and water, and is a necessary force driving global weather patterns. Within living systems, the presence of lipid-based membranes creates compartments that allow the selective concentration of water-soluble substances. For instance, mitochondrial membranes can create 2 distinct regions within the organelle the inner matrix and the inter-membrane space. Each of these sub-compartments has a specific composition and function, distinct from the adjoining spaces. The generation of order in this manner is one of the hallmarks of nearly every unit of the living world from organelles within a cell to entire organ systems and organisms.
However, this automatically means that ions, small molecules, proteins and other solutes have differential concentrations across lipid bilayers. Moreover, polar, charged or hydrophilic molecules cannot traverse biological membranes. While this is useful for maintaining the integrity of each compartment, it is equally necessary for molecules to move across membranes, along their concentration gradient, when needed.
Don’t Miss: Unit Test Answers For Edgenuity
What Is Facilitated Diffusion
Facilitated diffusion is the passive movement of molecules along the concentration gradient. It is a selective process, i.e., the membrane allows only selective molecules and ions to pass through it. It, however, prevents other molecules from passing through the membrane. The electric charge and pH helps in the diffusion across the membrane.
In living systems, the lipid based membrane creates compartments which allow the transport of a selective concentration of water-soluble substances. The ions, small molecules, proteins, and other solutes have different concentration across the membranes. Hydrophilic, polar or charged molecules cannot cross the membrane.
Importance Of Diffusion In Plants
The process of diffusion is important for the plants in the following ways:
Learn more about diffusion in plants, its types, importance and other related topics @ .
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Read Also: Eoc Algebra 1 Practice Test With Answers 2015