Remember Cation And Anion
There are a couple of simple mnemonics used to remember a cation is positive and an anion is negative. First, you can use the letters of the words. The t in cation is like a plus symbol. The letters in the word anion can stand for A Negative Ion. A pun to remember the difference is CATions are PAWSitive.
Denoting The Charged State
When writing the chemical formula for an ion, its net charge is written in superscript immediately after the chemical structure for the molecule/atom. The net charge is written with the magnitude before the sign that is, a doubly charged cation is indicated as 2+ instead of +2. However, the magnitude of the charge is omitted for singly charged molecules/atoms for example, the sodium cation is indicated as Na+ and not Na1+.
An alternative way of showing a molecule/atom with multiple charges is by drawing out the signs multiple times, this is often seen with transition metals. Chemists sometimes circle the sign this is merely ornamental and does not alter the chemical meaning. All three representations of Fe2+, Fe++, and Fe shown in the figure, are thus equivalent.
Monatomic ions are sometimes also denoted with Roman numerals, particularly in spectroscopy for example, the Fe2+ example seen above is referred to as Fe or FeII. The Roman numeral designates the formal oxidation state of an element, whereas the superscripted Indo-Arabic numerals denote the net charge. The two notations are, therefore, exchangeable for monatomic ions, but the Roman numerals cannot be applied to polyatomic ions. However, it is possible to mix the notations for the individual metal centre with a polyatomic complex, as shown by the uranyl ion example.
How To Remove An Anion Or Cation From Water
The two types of ion exchange units are water softeners and anion exchange devices. Water softeners remove cations and replace them with sodium. Anion exchange devices remove anions and replace them with chloride.
Mixed media ion exchange units remove both cations and anions. A typical mixture would be 60 percent cation exchange material and 40 percent anion exchange material. Units generally need to be regenerated in a central processing plant. Two-bed deionizers, using separate cation and anion vessels, can be backwashed to remove trapped particles.
Water softeners are the most widely used household water treatment devices. They remove scale-forming minerals on water heaters and soap film on sinks. Softening the water is sometimes called water conditioning.
Recommended Reading: Kuta Infinite Geometry
Predicting Cations And Anions Based On The Periodic Table
Whether an atom forms a cation or an anion depends on its position on the periodic table. Group 1A and 2A of the periodic table, alkali metals and alkaline earth metals respectively, always form cations. In contrast, Group 17A, which consists of halogens, always forms anions.
Most metals form cations, whereas most nonmetals form anions.
Is Oxygen A Cation Or An Anion
Many elements can take the form of either anions or cations depending on the situation. Oxygen often exists in a neutral state, but oxygen atoms tend to form anions by gaining two electrons. Atoms of other elements also tend to form anions, including nitrogen, chlorine, and fluorine, among others. By contrast, atoms of calcium, magnesium, aluminum, and sodium tend to lose electrons and form cations. Sodium chloridewhat we know as table saltis actually composed of an anion and a cation .
You May Like: What Does Cyte Mean
Examples Of Simple Anions
Five common examples of monoatomic anions are provided below.
- The fluoride anion, denoted by the chemical formula F.
- The chloride anion, denoted by the chemical formula Cl.
- The bromide anion, denoted by the chemical formula Br.
- The iodide anion, denoted by the chemical formula I.
- The sulfide anion, denoted by the chemical formula S2-.
Cations And Anions On The Periodic Table
Technically, any atom or molecule can form both cations and anions. For example, a hydrogen atom usually has a +1 oxidation state, but sometimes it gains an electron and has a -1 charge! That being said, metals usually form cations, while nonmetals usually form anions. To put it another way, elements on the left side of the periodic table tend to form cations, while those on the right side form anions. Noble gases are the exception. They are sufficiently stable that they dont form either anions or cations easily. Certain groups of the periodic table form characteristic ions:
- Alkali metals : +1 cations
- Alkaline earth metals : +2 cations
- Transition metals : Two or more oxidation states, usually differing by one. For example, copper forms +1 and +2 cations.
- Boron family : +1 or +3 cations
- Carbon family : -4 for carbon, but +2 going down the group
- Nitrogen family : +3 or +5
- Oxygen family : -2 for oxygen, but -2, +4, +6 going down the group
- Halogens : -1
- Noble gases : 0
Read Also: Elton John Children Biological
Examples Of Polyatomic Anions
Five common examples of polyatomic anions are provided below.
- The nitrate anion, denoted by the chemical formula NO3.
- The nitrite anion, denoted by the chemical formula NO2.
- The hydroxide anion, denoted by the chemical formula OH.
- The carbonate anion, denoted by the chemical formula CO32-.
- The sulfate anion, denoted by the chemical formula SO42-.
Effect Of Simultaneous Cation And Anion Doping On Optical Absorption And Photocatalysis
Co-doping into both anion and cation sites might be one of the most interesting ideas from the viewpoint of improving the optical absorptivity. It was predicted that -co-doping of rutile would greatly enhance the optical absorption properties of the material. While simple doping with nitrogen into oxygen sites gives an antiferromagnetic ground state, -co-doping leads to half-metallic ferromagnetism and a ferromagnetic ground state. These results were obtained using DFT and the plane-wave pseudopotential method . The findings have yet to be verified experimentally, for example, by using 57Co emission Mößbauer spectroscopy.
1 . The only quantity that can be measured experimentally is the difference between the fluxes of M-containing species directed toward the cathode and anode, that is, the net number of moles of M being transferred in one direction. For the passage of 1 F of charge across the cell, the net number of farads carried by the M constituent is its transference number, ti. The sum of the cation and anion transference numbers is equal to unity. The 0 ion pair does not influence the transference number.
-
Group 1: TubandtHittorf method and concentration cell techniques.
-
Group 2: Radiotracers pulsed field gradient nuclear magnetic resonance measurements electrophoretic nuclear magnetic resonance .
-
Group 3: Direct current polarization and alternating current impedance methods.
Recommended Reading: Is Paris Michael Jackson Biological Child
Polyatomic And Molecular Ions
Ionization is not limited to individual atoms polyatomic ions can also be formed. Polyatomic and molecular ions are often created by the addition or removal of elemental ions such as H+ in neutral molecules. For example, when ammonia, NH3, accepts a proton, H+, it forms the ammonium ion, NH4+. Ammonia and ammonium have the same number of electrons in essentially the same electronic configuration, but ammonium has an extra proton that gives it a net positive charge.
Transition Metals Cations Are Rule Breakers
| Charge = 1+ | |
|---|---|
| Zinc, Zn2+ | Iron, Fe3+ |
Unfortunately, transition metals dont have such a neat rule. Some transition metals can lose different number of electrons at different times, forming cations of different charges.
To avoid confusion, we sometimes indicate the charge of transition metal cation with the roman numeral behind its name. For example, iron cation has a charge of 2+.
Confusing the matter further, lead in Group IV can also form ions of different charges: lead and lead ions. However, only lead ions are featured in the O Level as they are way more common.
Also Check: Geometry: Homework Practice Workbook Answers
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Cations And Anions Form From Neutral Atoms
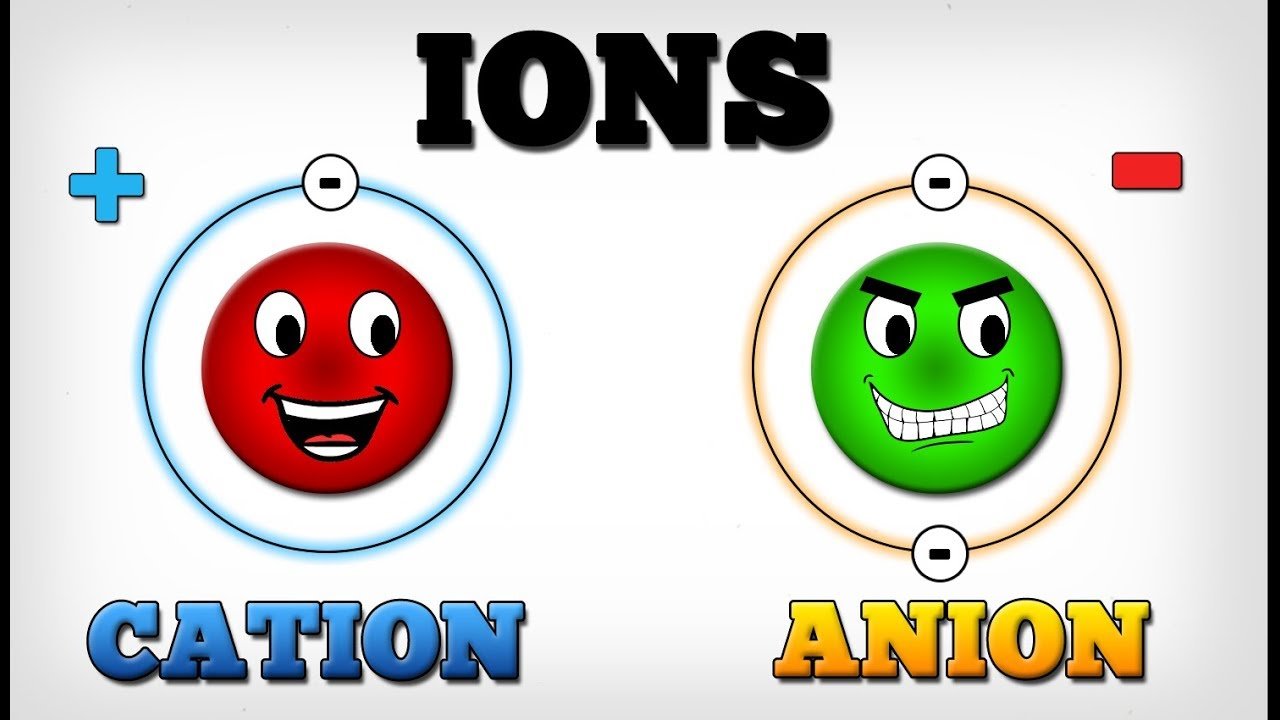
Every atom in its ground state is uncharged. It has, according to its atomic number, the same number of protons and electrons. Electrons are rather labile, however, and an atom will often gain or lose them depending on its electronegativity. The driving force for such gain or loss of electrons is the energetically optimal state of having a full valence shell of electrons. In such a state, the resulting charged atom has the electron configuration of a noble gas.
Addition of an electron will disrupt the proton-electron balance and leave the atom negatively charged. Removal of an electron will, conversely, leave the atom positively charged. These charged atoms are known as ions.
You May Like: Geometry Wars 3 Achievements
Can Cations And Anions Combine
Yes, cations and anions can combine. Because they are polar opposites, they attract each other. They form a binary compound. In this way, for example, sodium ions and chloride anions combine to make table salt.
However, ions that are not opposites repel one another, and, therefore, will not combine.
The Difference Between A Cation And An Anion
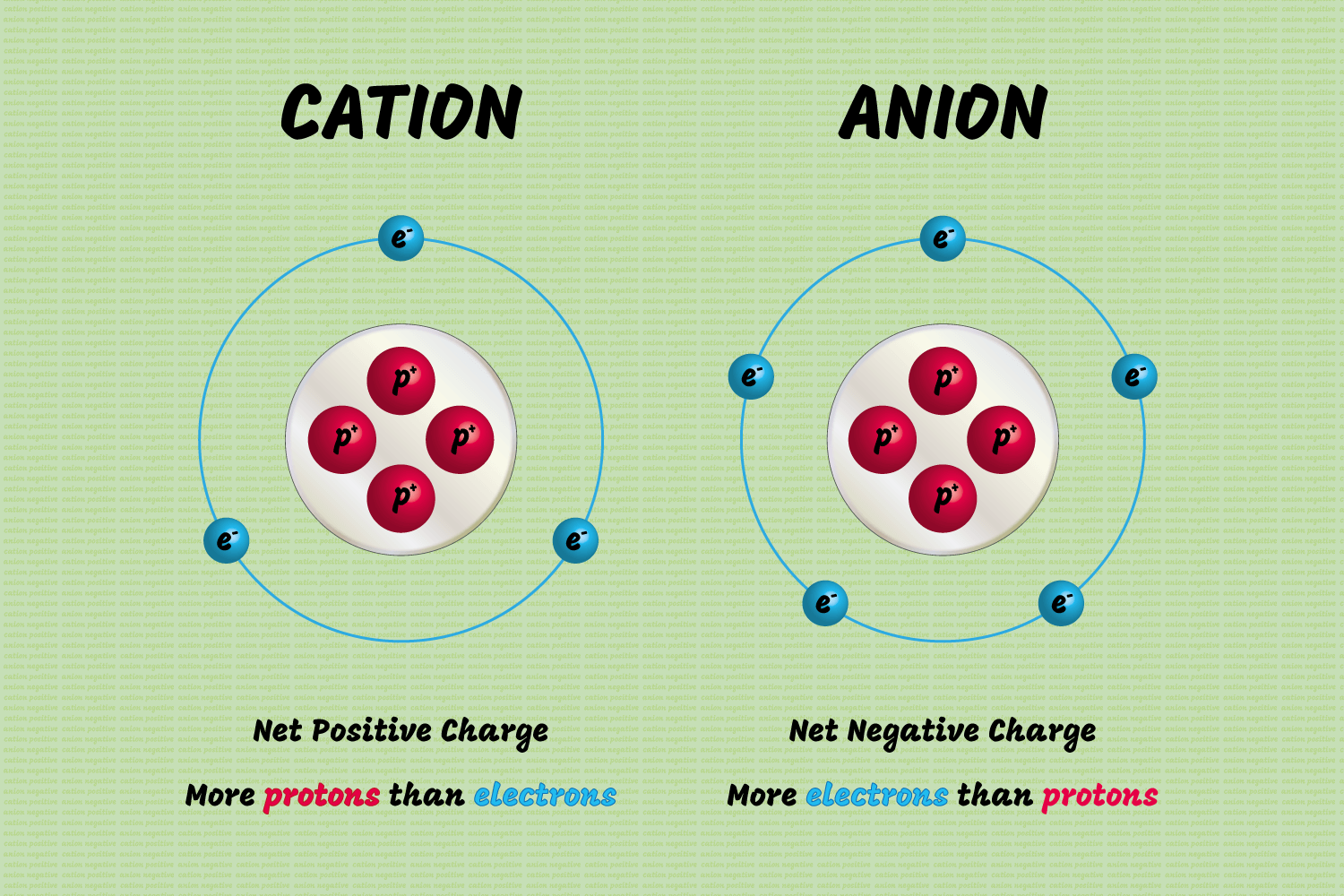
Cations and anions are both ions. The difference between a cation and an anion is the net electrical charge of the ion.
Ions are atoms or molecules which have gained or lost one or more valence electrons, giving the ion a net positive or negative charge. If the chemical species has more protons than electrons, it carries a net positive charge. If there are more electrons than protons, the species has a negative charge. The number of neutrons determines the isotope of an element but does not affect the electrical charge.
Don’t Miss: Geometry Segment Addition Worksheet Answer Key
Identifying The Anions And Cations Examples
1. Q1 is a simple salt. Carry out the following tests to identify the salt.
| Experiment | ||
|
||
|
|
|
Salt Q1 contains lead ion, Pb2+, and nitrate ion, NO3+. Q1 is lead nitrate.
2. Q2 is a salt containing one cation and one anion. Identify the ions from the following tests.
| Experiment |
Sorption Of Inorganic Cations
Unmodified hypercrosslinked polystyrene contains no specially introduced polar functional groups, although many publications report the presence of hydroxyl and carbonyl groups in these sorbents. Still, the amount of oxygen-containing groups is quite small, less than 1.5 mmol/g, since the value of 2.5% oxygen calculated from the elemental analysis of MN-200 sample as O% = 100 also includes noticeable amounts of adsorbed oxygen, carbon dioxide, water, as well as residues of the catalyst. Besides, incomplete combustion of the polymer that is prone to carbonization can easily contribute to the overestimation of the oxygen content.
In any case, the oxygen-containing groups cannot account for the retention of many ions of Hg2+ , which is characteristic of neutral hypercrosslinked polystyrene sorbents. The uptake of mercuric salts from weakly acidic aqueous solutions by the unfunctionalized materials proceeds very slowly. For Styrosorb 2 final equilibrium is established not earlier than within 8 days, leading to the incorporation of up to 200 mg of mercury per gram of the dry polymer. The approach to equilibrium is even slower on MN-200. Introduction of the same amount of mercury into this polymer would take no less then 12 days.
Figure 11.12. Sorption isotherms for mercury acetate from acetate buffer of pH 4 onto Styrosorb 2 with S = 1500 m2/g, MN-200, Styrosorb 1 , and Styrosorb 2 with S = 400 m2/g.
Philip G. Jessop, in, 2017
You May Like: Holt Geometry Homework And Practice Workbook Answer Key
Representing Charge On An Ion
The chemical formula of an ion is written first to represent the charge the charge that the ion holds. The positive charge is denoted by + in superscript and similarly the negative charge is denoted by the -symbol along with the magnitude of the charge that the ion holds .
For anions, magnitude of the charge is written first and then the sign . If the value is 1 then the magnitude of the charge is omitted. For example, sodium cations charge is +1 and hence it is represented as Na+.
What Is Anion And Cation In Chemistry
4.6/5AnionCationanions
Likewise, people ask, what is an anion in chemistry?
An anion is an ionic species having a negative charge. The chemical species may be a single atom or a group of atoms. An anion is attracted to the anode in electrolysis. Anions are typically larger than cations because they have extra electrons around them.
Also Know, what is cations and anions in chemistry? Cations and anions are formed when a metal loses electrons, and a nonmetal gains those electrons. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound, such as sodium chloride.
In this way, what is a cation in chemistry?
Cation, atom or group of atoms that bears a positive electric charge. See ion. Read More on This Topic. ion. Positively charged ions are called cations negatively charged ions, anions.
What is an anion example?
Anions are atoms or radicals , that have gained electrons. Since they now have more electrons than protons, anions have a negative charge. For example, chloride ions Cl- , bromide Br- , iodide I-. Anions are one of the two types of ions. The other type is called a Cation, having a positive charge.
Recommended Reading: How To Find Half Life In Chemistry
Confirmatory Tests For Fe2+ Fe3+ Pb2+ And Nh4+ Ions
Ammonium ion, NH4+
Method I:Heating an ammonium salt with a strong alkali Procedure: About 2 cm3 of ammonium chloride solution is poured into a test tube. About 4 cm3 of dilute sodium hydroxide solution is added to the test tube and the mixture is shaken well. The mixture is carefully heated and the gas liberated is tested with a piece of moist red litmus paper. Observation: The colourless gas evolved turns red litmus paper blue. Explanation: Heating an ammonium salt with an alkali produces ammonia gas. NH4+ + OH NH3 + H2O The alkaline ammonia gas turns red litmus paper blue.
Method II: Reacting with Nesslers reagent Procedure: About 2 cm3 of ammonium chloride solution is poured into a test tube. A dropper is used to add Nesslers reagent drop by drop to the solution. Any change that occurs is noted. Observation: A brown precipitate is formed. Explanation: Ammonium ion reacts with a complex ion in Nesslers reagent to produce a brown precipitate.
Iron ion, Fe2+
Procedure: About 2 cm3 of iron sulphate solution is poured into a test tube. A dropper is used to add potassiumhexacyanoferrate, K3Fe6 solution, drop by drop into the test tube. Any change that occurs is recorded. Observation: A dark blue precipitate is obtained. Explanation: The iron ion combines with a complex ion in the reagent to produce a dark blue precipitate. Fe2+ + Fe63- dark blue precipitate
Iron ion, Fe3+
Lead ion, Pb2+
| Cation | |
| Soluble in excess alkali to form dark blue solution. |
Some Examples Of The Most Common Nanoatomic Cation
Family |
|---|
| Iodine | Iodide anion |
It should be noted that it is more difficult to determine the number of electrons than the members of the transition metals . In fact, many of these elements lose a variable number of electrons so that they form two or more cations with different charges.
Also that the electric charge that an atom reaches is sometimes called: oxidation state. Many of the transition metal ions have different oxidation states. The following table shows some common transition metals that have more than one oxidation state.
Read Also: Common Core Worksheets 2nd Grade
What Is An Ion
The definition of an ion is a particle, atom, or molecule with an imbalance of electrical charge. Ions are charged. They contain different numbers of protons and electrons. Ions form when atoms move into a more stable electron configuration. Ions are identified by a superscript that shows the sign and size of the electric charge for example Ca+2. There are two types of ions: cations and anions.
A cation has a net positive electrical charge, which means it has more protons than electrons.
An anion has a net negative electrical charge, which means it has more electrons than protons.
How To Use Cation Vs Anion
Remember, cations are positive ionsthey are positively charged because they have lost one or more electrons and therefore have more protons than electrons.
Anions are negative ionsthey are negatively charged because they have gained one or more electrons and therefore have more electrons than protons.
When youre taking your chemistry test, just remember that cats are always a positive thing.
Also Check: Beth The Psychopathic Child Now
Ions: Points To Remember
- Atom is neutral, having equal number of protons and electrons whereas ion is electrically charged by removing or adding electrons from a neutral atom.
- Ion possesses an electrical charge. It can be negative or positive.
- It is basically an atom or a group of atoms having a negative/positive charge.
- When an ion loses an electron, it becomes a cation and when it gains an electron, it becomes an anion .
- Ions formed from single atoms are known as monoatomic ions.
- When ionic compounds are dissolved in water, they break into individual ions, resulting in dissociation. Each carries a polar charge.
- Ions which possess more than one atom are called polyatomic ions.
- Joining or breaking of two atoms lead to formation of ions.
- Ions are also formed when an atom loses or gains electrons to get a negative or positive charge.
- There are various types of anions based on their valency, such as monovalent , divalent , trivalent and tetravalent .