Solubility Trends In Group 17 Elements
The affinity of an elements atoms for one another can be quantified using entropy as a unit. For example, it takes less energy to bring gaseous H2S together than gaseous HCl since their molecules are more similar in size.
In contrast, it takes more energy to combine FCl since fluorine has a much smaller electron-cloud diameter than chlorine . By extension, mercury will take even less energy to form alloys with gold or platinum than with iron or nickel because its larger electron-cloud diameter better accommodates them.
What Is The Molecular Geometry Of H2co
According to VSEPR, the lowest energy can be obtained by minimizing repulsion between electron pairs around the central atom, resulting in the most stable geometry.
Throughout formalin, we will look at the electron pairs surrounding Carbon. We need the steric number of Carbon, which is the number of atoms bonded toward the nitrogen carbon depending on the number of lone pair of electrons on the central atom, to apply VSEPR. It is three for Carbon.
The molecular shape seems tetragonal flat when whole realms = three and lone pair = 0.
The molecular geometry of hydrogen chloride is considered to be in the shape of a trigonal pyramidal shape. The HCl molecule comprises one H atom bonded to one Cl atom. The bond length between the two atoms is about 154 pm. Bond angles are about 90°. The H and Cl atoms are held together by two covalent bonds within a molecular orbital.
The HCl molecule is held together by two strong, covalent bonds. The bond length between the two atoms is 154 pm. Bond angles are about 90°. The HCl molecule is held together by two covalent bonds within a molecular orbital. The H and Cl atoms are held together by two covalent bonds. The bond length between the two atoms is 154 pm. Bond angles are about 90°.
Formal Charges And Octet Rule:
Distinctive Lewis structures are feasible for an atom however the steadiest one is viewed as which observes the rule, with least partition of charges. One factor might overwhelm over the other for specific atoms.
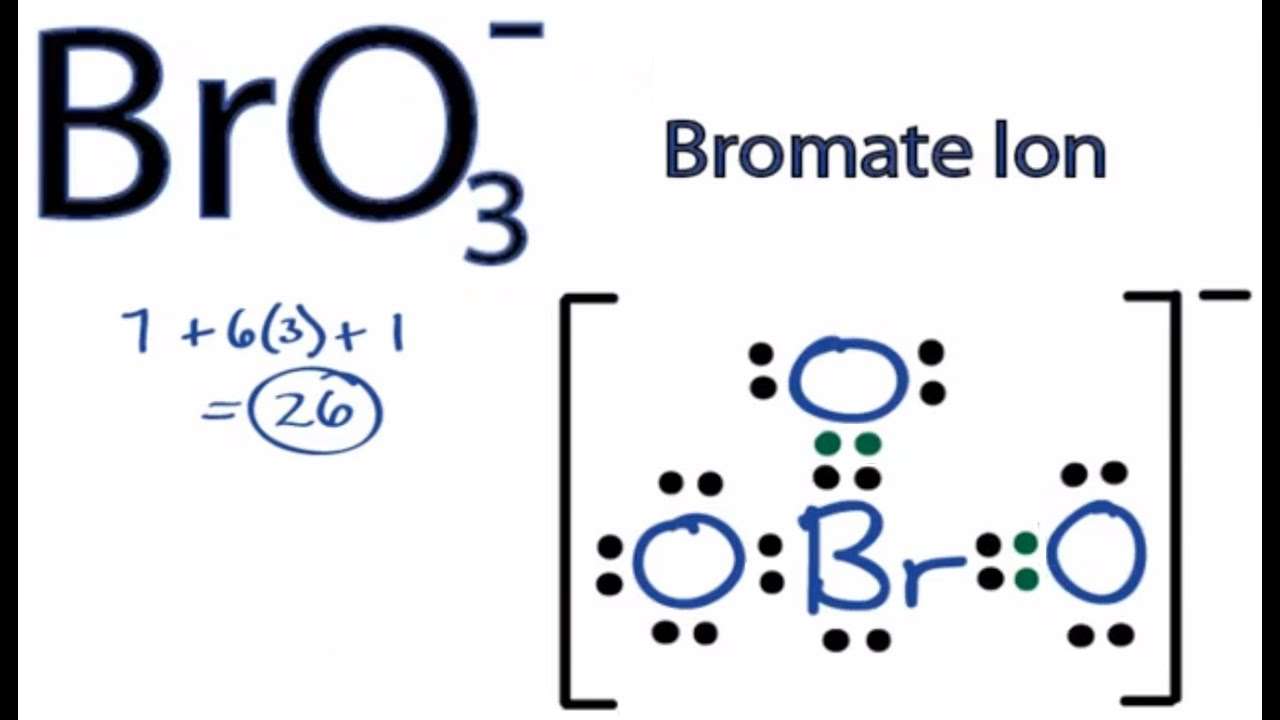
For BrO3BrO3 Br has 7 valence electrons, and O has 6. The conceivable Lewis structure which observes octet rule is:
In this design, the conventional charges are raised, Br has +1 formal charge, 2 of the O iotas have – 1 proper charge.
Another conceivable design what disrupts the octet guideline yet limits formal charges is:
Here, Br has 0 proper charge and just a single O has – 1. The octet rule isnt followed as Br has 12 electrons here rather than 8.
Also Check: 4 Main Areas Of Biological Contamination
Bromate Formation During Ozonating
Although ozonating produces minimal by-products, it does produce bromate when it combines with bromide ions in water. Bromide can be found in significant proportions in fresh water to create more than 10 ppb of bromate following ozonatingthe USEPAs maximum contamination threshold. Bromate formation can be reduced by lowering the pH of the water below 6.0, limiting ozone doses, and using ionisers.
FAQS
What is the charge of BrO3?
This molecule, known as a bromate molecule, has an oxidation number of -1. BrO3- should be typed appropriately. Because bromine has an oxidation number of +5, whereas oxygen has a standard oxidation number of -2, it has a negative net charge.
What is the name for MG BrO3 2?
Magnesium Bromate Mg2 Molecular Weight EndMemo.
What is the name of BrO2?
BrO2 Bromine dioxide is an inorganic radical and a bromine oxide.
What charge is cro4?
Chromate. Chromate is an ion that contains one chromium atom and four oxide atoms. Its formula is CrO4. Its overall charge is -2.
What color is cro4?
| color of cro4-2 in solution | yellow |
What is the Lewis structure of Bro3 ? Lewis structure of BrO3- is a bromine based oxo anion, it is a monovalent inorganic anion. It is denser than water. Bromate particle is shaped when ozone responds with bromide anion.
What Is The Molecular Geometry Of Icl2
In chemistry, geometry refers to the spatial arrangement of the atoms that make up a molecule or a crystal. The three-dimensional geometry of a molecule or crystal is defined by the locations of its atoms and its stereochemistry. The most common geometries are face-centred cubic, body-centred cubic, and hexagonal close-packed arrangements.
The molecular formula of icl2 is i. The molecular geometry of icl2 is c. The shape of a molecule can be thought of as the shape of a solid object. The shape of icl2 is a. The bond angle of icl2 is t. The bond angle of icl2 is p. A bond refers to the angle formed by two bonded atoms. icl2 has an atomic radius of r.
Th distance between the nucleus and the outermost electrons is measured in atomic radius. The atomic radius of icl2 is r. The bond length of icl2 is l. The bond length of icl2 is l. The bond length is the distance between two bonded atoms. The bond length of icl2 is l. The Lewis structure of icl2 is i. The polarizability of icl2 is a.
The polarizability measures how much an atom or molecule is attracted to a magnet. The dipole moment of icl2 is p. The dipole moment measures how much the molecules are attracted to an external electric field. The dipole moment of icl2 is p. The volume of icl2 is V.
Read Also: Example Of Movement In Geography
What Is The Molecular Geometry Of H2s
The electronic configuration of hydrogen atoms in H2S is
Looking at valence bond theory, we know that a single H atom forms two bonds with each S atom to make a molecule. Each bond contains two electrons, so there are four bonding electrons between H and S atoms. The two lone pairs on each S atom overlap with other teams to form six shared electron pair bonds around a central ionic core or molecule consisting of four ions since S has a valence number +4 one for each pair 2-electron bonds between it and its neighbours.
List Of Lewis Structures
|XeF4|||||||
Formation of hydrated electron and BrO3 radical from laser photolysis of BrO3- aqueous solution
One of the results of laser photolysis of bromate aqueous solution was hydrated electron, as well as BrO3 radical, with an initial quantum yield of 10-2 found. O2, OH radical, and the parent molecule BrO3- can quickly scavenge the hydrated electron generated. 3.0109 d mol-1 s was found to be the rate constant for the reaction of eaq- with BrO3-.
Formation of Bro 3
As written, neutral BrO3 does not exist, at least not for the time being. The bromate ion, BrO3-, has a trigonal pyramidal shape. We have the following core atom: Br after completing the Lewis structure of Bromate Ion and using the VESPR method to determine the molecular geometry:
The center atom has seven electrons.
3 electrons contribute to the 3 oxygens:
The center atom for the bonding is provided by electrons: -2. Ion charge : 1.9 total electrons.
However, if we divide 9 by 2.
The possible molecular geometries are:
Step 1: Bromine is less electronegative than oxygen so Br is the central atom .
Step 2: We need to determine the total number of valence electrons present in the molecule:
Group Valence Electrons
Br 7A 1 × 7 e = 7 e
O 6A 3 × 6 e = 18 e
From -1 charge: + 1e
1586444545487.jpg)
Bromate
3, is a bromine -based oxoanion. A bromate is a chemical compound that contains thision. Examples of bromates include sodium bromate, (NaBrOand potassium bromate , (KBrO
3 + 2 Br
Read Also: What Does Ml Stand For In Chemistry
What Is The Molecular Geometry Of Bro3
Molecule geometry is the three-dimensional arrangement of the atoms that constitute a molecule. The Lewis structure of the molecule represents the three-dimensional coordinates of the atoms. The molecule geometry can be described by the number of atoms , the bond length , the bond angle and the dihedral angle.
The anion with the chemical formula BrO 4 is the perbromate ion in chemistry. This is also a bromine oxyanion, the conjugate base of perbromic acid, with a bromine oxidation state +7.
BrO3- has a trigonal pyramidal molecular geometry. BrO3-lewis structure contains 16 lone pairs electrons and ten bonded pairs electrons. BrO3- has a tetrahedral electron geometry.
Drawing The Lewis Structure For Bro3
Video: Drawing the Lewis Structure for BrO3-
In the BrO3- Lewis structure Bromine is the least electronegative and goes in the center of the dot structure. Remember that Bromine can hold more than 8 valence electrons and have an expanded octet.
For the BrO3- Lewis structure, calculate the total number of valence electrons for the BrO3- molecule. After determining how many valence electrons there are in BrO3-, place them around the central atom to complete the octets. Be sure to use the number of available valence electrons you found earlier.
For the Lewis structure of BrO3- you should take formal charges into account to find the best Lewis structure for the molecule. Also note that you should put the BrO3- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
It is helpful if you:
- Try to draw the BrO3- Lewis structure before watching the video.
- Watch the video and see if you missed any steps or information.
- Try structures similar to BrO3- for more practice.
Also Check: Exponential Growth And Decay Common Core Algebra 1 Homework Answer Key
Put Two Electrons Between The Atoms To Represent A Chemical Bond
Now in the above sketch of BrO3 molecule, put the two electrons between each bromine atom and oxygen atom to represent a chemical bond between them.
These pairs of electrons present between the Bromine and Oxygen atoms form a chemical bond, which bonds the bromine and oxygen atoms with each other in a BrO3 molecule.
What Number Of Valence Electrons In Bro3
In the wake of deciding the number of valence electrons there are in BrO3-, place them around the focal particle to finish the octets. Make certain to utilize the quantity of accessible valence electrons you found before.
For the Lewis construction of BrO3-you should consider formal charges to track down the best Lewis structure for the particle. Additionally note that you should put the BrO3-Lewis structure in sections with as 1 outwardly to show that it is a particle with a negative one charge.
Read Also: 100 Day Countdown To The 5th Grade Math Fsa 1-50 Answer Key
Lewis Structure Of Bro3
Ready to learn how to draw the lewis structure of BrO3- ion?Awesome!Here, I have explained 6 simple steps to draw the lewis dot structure of BrO3- ion .So, if you are ready to go with these 6 simple steps, then lets dive right into it!
Lewis structure of BrO3- contains two double bonds and one single bond between the Bromine atom and Oxygen atom. The Bromine atom is at the center and it is surrounded by 3 Oxygen atoms . The Bromine atom has 1 lone pair. And the single bonded oxygen atom has -1 formal charge.
Lets draw and understand this lewis dot structure step by step.
Some Important Facts About Bro3

Bromine gas is converted to bromate via the photoactivation process. But in the laboratory bromate can be prepared by dissolving bromine into a concentrated solution of potassium hydroxide solution.
Br + 2 OH = BrO + H2O
3BrO = BrO + 2Br
The main process of formation of bromate is a reaction between ozone and bromide.
O3 + Br = BrO3
Bromate-containing water is bad for human life.
You May Like: Algebra 1 Eoc Fsa Practice Test No Calculator Portion Answers
S Of Drawing Lewis Structure Of Bro3
When we draw a lewis structure, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion. Because BrO3- ion is an ion and there are four atoms, we will have more steps than drawing a simple molecule or ion. However, those all steps are mentioned and explained in detail in this tutorial to improve your knowledge about lewis structure.
What Is The Molecular Geometry Of Clo2
Clo2, also known as dioxygenyl dichloride, is a chemical compound with the formula ClO2. The combination is a colourless, volatile helpful liquid as a bleach and dioxygen source in chemical reactions. Clo2 was the first ionic substance prepared artificially by the English chemist Sir Humphry Davy in 1808. Davy had previously isolated the element oxygen in 1795.
Clo2 has a molecular geometry of CClF, which is a trigonal pyramidal molecular geometry. A trigonal pyramidal molecular geometry is a molecular geometry. The central atom has three bonds pointing towards the apex and three bonds away from the peak.
The three bonds meaning away from the height, are the equatorial bonds. The three bonds pointing towards the apex are the axial bonds. The apex represents the central atoms lone electron pair. The three bonds meaning away from the apex are shorter than the three bonds pointing towards the apex.
Don’t Miss: How Do You Find Displacement
What Is The Molecular Geometry Of Brf5
Hydrogen sulphide has a linear molecular geometry, meaning that its molecular bonds run along one straight line. This molecule also has two unpaired electrons. Chlorine trifluoride has a linear molecular geometry with three unpaired electrons in its outer shll.
Brmine pentafluoride has a linear structure with five unpaired electrons on its outer surface. Chloroethane and hydrocyanic acid have trigonal pyramidal structures these molecules have three bonds running between each pair of atoms, creating an overall pyramid shape. Two different atoms are bonded at each of two opposite corners.
Calculate The Total Number Of Valence Electrons
Here, the given ion is BrO3- . In order to draw the lewis structure of BrO3- ion, first of all you have to find the total number of valence electrons present in the BrO3- ion..
So, lets calculate this first.
Calculation of valence electrons in BrO3-
- For Bromine:
Bromine is a group 17 element on the periodic table.
Hence, the valence electrons present in bromine is 7 .
- For Oxygen:
Oxygen is a group 16 element on the periodic table.
Hence, the valence electron present in oxygen is 6 .
Hence in a BrO3- ion,
Valence electrons given by Bromine atom = 7 Valence electrons given by each Oxygen atom = 6 Electron due to -1 charge, 1 more electron is addedSo, total number of Valence electrons in BrO3- ion = 7 + 6 + 1 = 26
Also Check: Beth Thomas Psychopathic Child
Check Whether The Central Atom Has Octet Or Not
In this step, we have to check whether the central atom has an octet or not.
In simple words, we have to check whether the central Bromine atom is having 8 electrons or not.
As you can see from the above image, the central atom , is having 8 electrons. So it fulfills the octet rule and the bromine atom is stable.
What Is The Shape Of Bro3
pyramidal
. Accordingly, what is the structure of BrO3?
BrO3– has a total of 26 valence electrons. In the Lewis structure, Br is placed in the center since it is lower in electronegativity. With all single bonds connecting the atoms, the formal charge of the O atoms are each -1 while the Br is 2.
Also Know, what is the shape of ICl4? With five nuclei, the ICl4 ion forms a molecular structure that is square planar, an octahedron with two opposite vertices missing.
Likewise, is BrO3 trigonal planar?
VSEPR geometry of BrO3– But if we divide 9 with 2 in order to find the -bonding pairs, we get 4,5. Because after searching the molecular geometry of BrO3– it says that it is trigonal pyramid.
What is the hybridization of BrO3?
In BrO3 – central metal atom is ‘Br’ and it has 3 -sigma bond and 1- lone pair present ,therefore it has sp3 hybridisation and trigonal shape and in ‘HOCl’ central metal atom is ‘O’ and it has 2- sigma bond and 2-lone pair therefore it has Sp3 hybridisation and angular shape.
Also Check: What Does Similar Figures Mean In Math
What Is Vsepr Of Bro3
It’s electron geometry is tetrahedral but it’s molecular geometry is pyramidal. Bond angles are less than 109.5 degrees. Because of the unpaired electrons , it is polar. Partial positive charges on the bromine with unpaired electrons, and partial negative on the oxygen bonds. Central atom hybridization=sp3. For a picture, I would suggest an internet search for VSEPR AB3E.Make the Lewis Dot structure and you will see that one pair of electrons is non-bonding. The repulsion between electron pairs is strongest between two non-bonding PAIRS of e-. But, the next strongest repulsion is between a bonding pair and a non-bonding pair. In the case of BrO3, the non-bonding pair is surrounded by two bonding pairs of e-. That is why the model shows the bonds angling away and down from the central atom instead of straight out.Draw an arrow up from the Br atom through the non-bonded pair of e- to represent the most repulsive pair of e-. This is the “E” in AB3E.Draw arrows out through the other 3 bonded pairs of e- and make them a little shorter to remind you why this molecular geometry is pyramidal in shape rather than merely tetrahedral. You should have 4 arrows pointing away from the Br one bigger than the other three to represent the unbonded e-.
Check The Stability And Minimize Charges On Atoms By Converting Lone Pairs To Bonds
Because every atom has charges and having a charge like +2 ion iodine atom, above structure has to consider as not stable. Therefore, we should try to reduce charges by converting lone pairs to bonds to find the most stable structures.
As below, we convert lone pairs of oxygen atoms to bonds in two steps.
You can see, charges has been reduced in new structure than previous structures. There is only -1 charge on one oxygen atom.
Questions
Read Also: What Does Math.floor Do In Java