To Identify Unknown Chemicals
Example 4: To Determine Molecular Formula from Mass Percentages
It is known that a chemical compound contains 52.14;% carbon, 13.13;% hydrogen, and 34.73;% oxygen. The molar mass of the chemical is also known; it is 46.069;g;mol1.
Step 1: First, we need to convert the mass percentages into moles. Consider 100;g of the compound. So, it has 52.14;g of carbon, 13.13;g of hydrogen, and 34.73;g of oxygen.
To now, the respective moles are nC;=;52.14;÷;12.011;=;4.341;mol, nH;=;13.13;÷;1.008;=;13.026;mol, and nO;=;34.73;÷;15.999;=;2.170;mol.
Step 2: Now, we have to take the mole to mole ratio of each element such that the divisor is the smallest number.
Thus, we have nC;:;nH;:;nO;=;2;:;6;:;1.
Step 3: We can determine the empirical formula from the mole ratios. So, the empirical formula is C2H6O. And the molar mass of the empirical mass is as follows:
Now, the empirical mass and the molar mass are the same; hence, the empirical and molecular formula must be the same, which is C2H6O.
The compound can be ethanol, CH3CH2OH or dimethyl ether, CH3OCH3.
Determining The Empirical Formula Of Penicillin
Just as the empirical formula of a substance can be used to determine its percent composition, the percent composition of a sample can be used to determine its empirical formula, which can then be used to determine its molecular formula. Such a procedure was actually used to determine the empirical and molecular formulas of the first antibiotic to be discovered: penicillin.
Antibiotics are chemical compounds that selectively kill microorganisms, many of which cause diseases. Although antibiotics are often taken for granted today, penicillin was discovered only about 80 years ago. The subsequent development of a wide array of other antibiotics for treating many common diseases has contributed greatly to the substantial increase in life expectancy over the past 50 years. The discovery of penicillin is a historical detective story in which the use of mass percentages to determine empirical formulas played a key role.
Penicillium mold is growing in a culture dish; the photo shows its effect on bacterial growth. In this photomicrograph of Penicillium, its rod- and pencil-shaped branches are visible. The name comes from the Latin penicillus, meaning paintbrush.
The empirical formula of penicillin G is therefore C16H17N2NaO4S. Other experiments have shown that penicillin G is actually an ionic compound that contains Na+ cations and anions in a 1:1 ratio. The complex structure of penicillin G ) was not determined until 1948.
Given: percent composition
Percent Composition Of Water
Another simple example is finding the mass percent composition of the elements in water, H2O.
First, find the molar mass of water by adding up the atomic masses of the elements. Use values from the periodic table:
- H is 1.01 grams per mole
- O is 16.00 grams per mole
Get the molar mass by adding up all the masses of elements in the compound. The subscript after the hydrogen indicates there are two atoms of hydrogen. There is no subscript after oxygen , which means only one atom is present.
- molar mass = + 16.00
- molar mass = 18.02
Now, divide the mass of each element by the total mass to get the mass percentages:
mass % H = / 18.02 x 100%mass % H = 11.19%
The mass percentages of hydrogen and oxygen add up to 100%.
Don’t Miss: What Is The Molecular Geometry Of Ccl4
From Empirical Formula To Molecular Formula
The empirical formula gives only the relative numbers of atoms in a substance in the smallest possible ratio. For a covalent substance, chemists are usually more interested in the molecular formula, which gives the actual number of atoms of each kind present per molecule. Without additional information, however, it is impossible to know whether the formula of penicillin G, for example, is C16H17N2NaO4S or an integral multiple, such as C32H34N4Na2O8S2, C48H51N6Na3O12S3, or n, where n is an integer. ).
Consider glucose, the sugar that circulates in our blood to provide fuel for the body and brain. Results from combustion analysis of glucose report that glucose contains 39.68% carbon and 6.58% hydrogen. Because combustion occurs in the presence of oxygen, it is impossible to directly determine the percentage of oxygen in a compound by using combustion analysis; other more complex methods are necessary. Assuming that the remaining percentage is due to oxygen, then glucose would contain 53.79% oxygen. A 100.0 g sample of glucose would therefore contain 39.68 g of carbon, 6.58 g of hydrogen, and 53.79 g of oxygen. To calculate the number of moles of each element in the 100.0 g sample, divide the mass of each element by its molar mass:
Once again, the subscripts of the elements in the empirical formula are found by dividing the number of moles of each element by the number of moles of the element present in the smallest amount:
\ + \left + \left = 30.026 g \label\]
Chemical Equations And Calculations
Reaction information is shown using word and symbol equations. Mass is conserved in chemical reactions. We can predict the masses of products and reactants involved in chemical reactions as no atoms are made or destroyed in chemical reactions. Relative atomic masses can be used to find the relative formula mass of a compound.
You May Like: Lesson 9.5 Geometry Answers
Density: A Further Investigation
We know all of density’s components, so let’s take a closer look at density itself. The unit most widely used to express density is g/cm3 or g/mL, though the SI unit for density is technically kg/m3. Grams per centimeter cubed is equivalent to grams per milliliter . To solve for density, simply follow the equation d = m/v. For example, if you had a metal cube with mass 7.0 g and volume 5.0 cm3, the density would be
Sometimes, you have to convert units to get the correct units for density, such as mg to g or in3 to cm3.
Density can be used to help identify an unknown element. Of course, you have to know the density of an element with respect to other elements. Below is a table listing the density of a few elements from the Periodic Table at standard conditions for temperature and pressure, or STP corresponding to a temperature of 273 K and 1 atmosphere of pressure.
| Element Name and Symbol | |
|---|---|
| 22.6 | 76 |
As can be seen from the table, the most dense element is Osmium with a density of 22.6 g/cm3. The least dense element is Hydrogen with a density of 0.09 g/cm3.
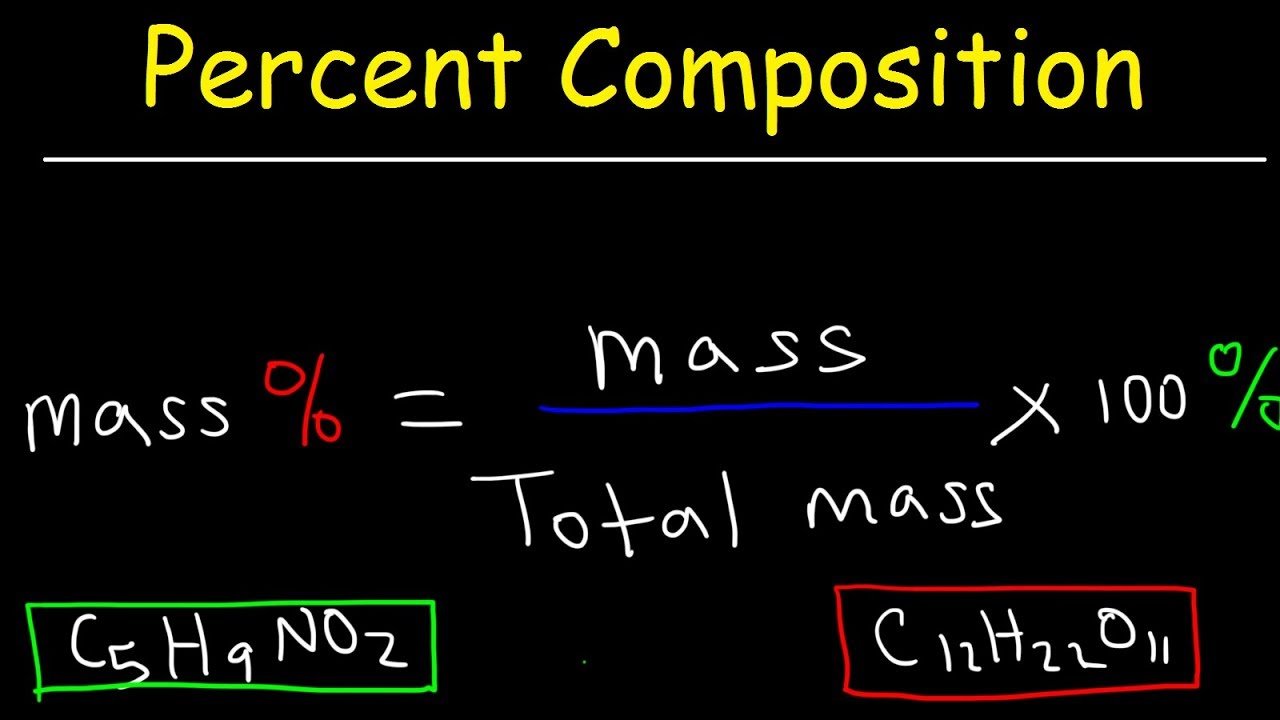
Percent Composition By Mass
Percent composition is calculated from a molecular formula by dividing the mass of a single element in one mole of a compound by the mass of one mole of the entire compound. This value is presented as a percentage.
Percentage Composition YouTube: This video shows how to calculate the percent composition of a compound.
For example, butane has a molecular formula of C4H10. Butanes percent composition can be calculated as follows:
- Mass of H per mol butane:;10\text\cdot\frac}} = 10.079\text
- Mass of C per mol butane:;4\text\cdot\frac}}=48.044\text
- Mass percent H in butane: \frac}}\cdot100=17.3\%\text
- Mass percent C in butane: \frac }}\cdot100=82.7\%\text
Therefore, the atomic composition of butane can also be described as 17.3% hydrogen and 82.7% carbon, and, as expected, these values sum to 100%.
Butane: The structural formula of butane.
In practice, this calculation is often reversed. Mass percents can be determined experimentally via elemental analysis, and these values can be used to calculate the empirical formula of unknown compounds. However, this information is insufficient to determine the molecular formula without additional information on the compounds molecular weight.
Recommended Reading: Introduction To Exponential Functions Common Core Algebra 1 Homework Answer Key
Formula For Percent Comp By Mass
This is a general formula for finding percent composition.
The molar mass of the element means the molar mass for the total number of that element in the compound. For example, in H2O2 we would find the molar mass of H and then multiply by “2” since there are two Hydrogen atoms.
More Help and Practice:
Solving For Mass Percent When Given Masses
Recommended Reading: Imagine Math Login
Practice Percent Comp By Mass
How to Practice Mole Conversions:
- Find the molar mass for each group of elements in the compound .
- Find the molar mass for the entire compound.
- Divide the mass for total mass for each element by the mass of the entire compound. Multiply by 100 to get percent.
Remember, you’re finding the percent composition of each element in the compound, one element at a time.
Examples Of Mass Percent Problems In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
This is a worked example problem showing how to calculate mass percent composition. Percent composition indicates the relative amounts of each element in a compound. For each element, the mass percent formula is:
% mass = / x 100%
mass percent = x 100%
The units of mass are typically grams. Mass percent is also known as percent by weight or w/w%. The molar mass is the sum of the masses of all the atoms in one mole of the compound. The sum of all the mass percentages should add up to 100%. Watch for rounding errors in the last significant figure to make sure all the percentages add up.
Don’t Miss: Ccl4 Dot Structure
Percent Composition: What Is It And How To Calculate It From A Chemical Formula
How to calculate percent composition
Percent means one part in a 100 or part in a whole. Therefore, percent composition of a compound is the percent by mass of each element in the total mass of a compound. Another way of saying the same thing is how much in percent each element contributes to the total mass of a compound. The higher the percentage composition, the higher the mass of the element present in the compound. Mathematically, we can express percentage composition as:
Calculating percentage composition
Now, lets use the above formula to calculate the percentage composition of each element in water H2O. From the chemical formula of water, we know that 1 mole of water contains 2 mol of hydrogen, H and 1 mol of oxygen, O. That is, we are assuming that:
1 mol of H2O contains 2 mol of H and 1 mol of O. But we do know that the molar mass of hydrogen is about 1.01 g/mol and the molar mass of oxygen is about 16.00 g/mol. Therefore, we can calculate the mass of 1 mol of water, H2O as:
Calculating percentage composition of H in waterCalculating the percentage composition of oxygen in water
From the calculation, you can see that the percentage composition of hydrogen and oxygen in water are 11.21% and 88.79% respectively. If you add these values together, you will notice they sum up to 100%. This means regardless of whether water is from mars or from earth, once it is pure water, its composition is fixed and will always consist of 11.21% H and 88.79% O.
Notice!
How To Calculate Dilutions
You dilute a solution whenever you add solvent to a solution. Adding solvent results in a solution of lower concentration. You can calculate the concentration of a solution following a dilution by applying this equation:
MiVi = MfVf
where M is molarity, V is volume, and the subscripts i and f refer to the initial and final values.
Example:How many milliliters of 5.5 M NaOH are needed to prepare 300 mL of 1.2 M NaOH?
Solution:5.5 M x V1 = 1.2 M x 0.3 LV1 = 1.2 M x 0.3 L / 5.5 MV1 = 0.065 LV1 = 65 mL
So, to prepare the 1.2 M NaOH solution, you pour 65 mL of 5.5 M NaOH into your container and add water to get 300 mL final volume
You May Like: Ccl4 Molecular Geometry
What Is Meant By Percent Composition
In chemistry, percent composition of a compound is defined as the amount of each element of a compound divided by the total amount of individual elements in a compound which is multiplied by 100. The percent composition of an element is given by the formula:%CE = 100%CE is the percent composition of an element
| Free Online Calculators |
Mass Percent Composition From A Chemical Formula
Learning Objectives
- Determine the percent composition of each element in a compound from the chemical formula.
The percent composition of a compound can also be determined from the formula of the compound. The subscripts in the formula are first used to calculate the mass of each element in one mole of the compound. This;is divided by the molar mass of the compound and multiplied by \.
The percent composition of a given compound is always the same, given that;the compound is pure.
Example \
Dichlorine heptoxide \\) is a highly reactive compound used in some organic synthesis reactions. Calculate the percent composition of dichlorine heptoxide.
Solution
| Calculate the percent composition of dichlorine heptoxide \\). | |
|---|---|
| Identify the “given” information and what the problem is asking you to “find.” |
Given : Cl2O7 Find: % Composition |
| List other known quantities. |
Mass of Cl in 1 mol Cl2O7 , 2 Cl : 2 x 35.45 g = 70.90 g Mass of O in 1 mol Cl2O7 , 7 O: 7 x 16.00 g = 112.00 g Molar mass of Cl2O7 = 182.90 g/mol |
| Cancel units and calculate. |
Calculate the percent by mass of each element by dividing the mass of that element in 1 mole of the compound by the molar mass of the compound and multiplying by \. |
| Think about your result. | The percentages add up to \. |
The sum of the two masses is \, the mass of the sample size.
Exercise \
Barium fluoride is a transparent crystal that can be found in nature as the mineral frankdicksonite. Determine the percent composition of barium fluoride.
Read Also: Exponential Growth And Decay Algebra 1 Worksheet
How To Calculate Percentage Composition
All we need is the molecular formula and the molar mass of each element present in a compound to determine percentage composition. It is the percentage ratio of the total mass of an element to the total mass of the compound.
Now, the total mass of each element is the molar mass of that element times the number of atoms of that element. And the molar mass of a compound is the sum of the molar mass times the number of atoms of each element.
Consider an generic molecular formula: AxByCz. So, the total mass of A per mole of AxByCz is x;MA. And the molar mass of AxByCz is MAxByCz;=;x;MA;+;y;MB;+;z;MC.
Mass percentage of A is as follows:
Here, MA, MB, and MC are the molar mass of A, B, and C.
Similarly, for B and C,
Example 1: Water
Let take a real example. Water, the most abundant compound in our solar system, is composed of hydrogen and oxygen. The molecular formula is H2O. One mole of water has two moles of hydrogen atoms and one mole of oxygen atoms. The molar mass MH2O of water is as follows:
The percentage composition of hydrogen in water is calculated as follows:
Similarly, for oxygen,
Note: Sum of %;composition of each element is always equal to 100;%.
Example 2: Copper Bromide
Consider an another example of copper bromide CuBr2. The molar mass of CuBr2 is as follows:
The percentage composition of copper in copper bromide is calculated as follows:
Similarly, for bromine,
Percent Composition Of Compounds
- Translate between a;molecular formula of a compound and its percent composition by mass
Key Points
- The atomic composition of chemical compounds can be described in a variety of ways, including molecular formulas and percent composition.
- The percent composition of a compound is calculated with the molecular formula: divide the mass of each element found in one mole of the compound by the total molar mass of the compound.
- The percent composition of a compound can be measured experimentally, and these values can be used to determine the empirical formula of a compound.
Term
- percent by massThe fraction, by weight, of one element of a compound.
The atomic composition of chemical compounds can be described using a variety of notations including molecular, empirical, and structural formulas. Another convenient way to describe atomic composition is to examine the percent composition of a compound by mass.
Read Also: Ccl4 Lewis Structure
Composition Of Chemical Compounds
chemical formulaismolecular formulaformula weightismolecular weightglucose
Ionic substances are not chemically bonded and do not exist as discrete molecules. However, they do associate in discrete ratios of ions. Thus, we can describe their formula weights, but not their molecular weights. Table salt , for example, has a formula weight of: