What Instrument Is Used To Measure The Relative Masses Of Atoms
At present, mass spectrometry is the technique used to measure the relative masses of atoms and their percentage abundance in nature. In a mass spectrometer, atoms interact with a magnetic field and separate according to their mass to charge ratio. As they separate according to this ratio, their percentage abundance and relative atomic masses can be calculated. Lets use the following example to illustrate how the relative mass of an atom is calculated using carbon-12 as the standard.
Set Up The Relative Abundance Problem
Use the following formula for relative abundance chemistry problems:
+ = M
When the information is placed into the equation, it looks like this:
14.003x + 15.000 =14.007
Why the equation can be set up this way: Recall that the sum of these two isotopes will equal 100 percent of the total nitrogen found in nature. The equation can be set up as a percent or as a decimal.
As a percent, the equation would be: + = 100, where the 100 designates the total percent in nature.
If you set the equation as a decimal, this means the abundance would be equal to 1. The equation would then become: x + = 1. Note that this equation is limited to two isotopes.
Chemical And Molecular Properties
A neutral atom has the same number of electrons as protons. Thus different isotopes of a given element all have the same number of electrons and share a similar electronic structure. Because the chemical behavior of an atom is largely determined by its electronic structure, different isotopes exhibit nearly identical chemical behavior.
The main exception to this is the kinetic isotope effect: due to their larger masses, heavier isotopes tend to react somewhat more slowly than lighter isotopes of the same element. This is most pronounced by far for protium , and tritium , because deuterium has twice the mass of protium and tritium has three times the mass of protium. These mass differences also affect the behavior of their respective chemical bonds, by changing the center of gravity of the atomic systems. However, for heavier elements, the relative mass difference between isotopes is much less so that the mass-difference effects on chemistry are usually negligible. There is also an equilibrium isotope effect.
ZNZNZ
Also Check: Does Kamala Harris Have Children
How To Calculate The Number Of Atoms Given The Grams And Atomic Mass Units
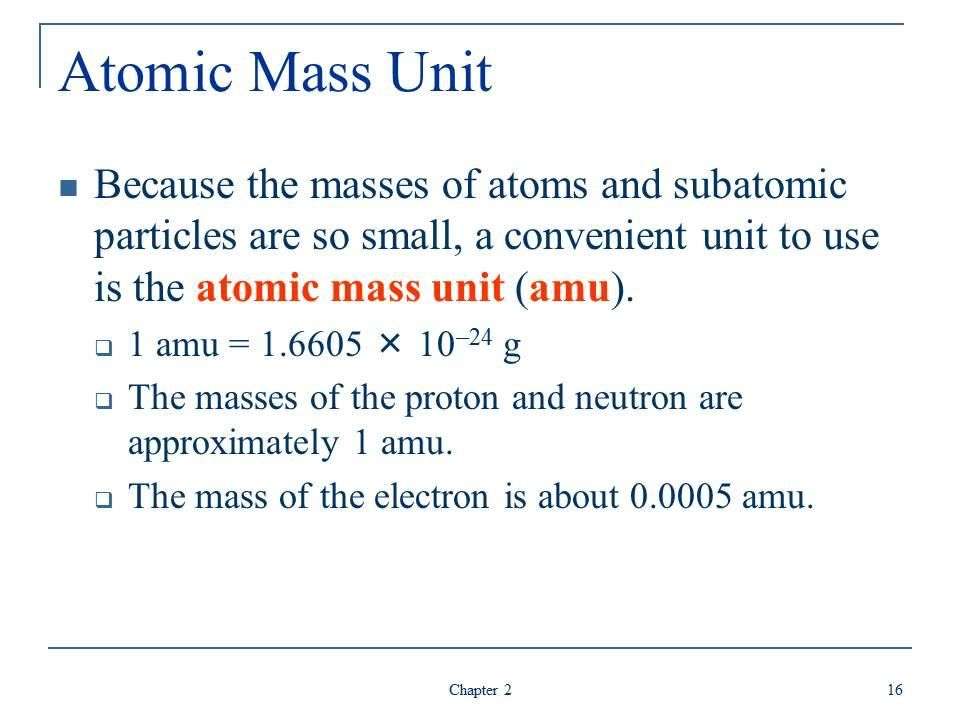
In the periodic table of elements, you’ll see each element’s atomic weight listed. Scientists use atomic mass units to describe the mass of atoms, so think of atomic weights in terms of amus. Avogadro’s constant — 6.02 x 10^23 — describes the number of atoms in a mole of an element. Weighing a sample of an element gives you its mass in grams. If you have all three pieces of information — atomic weight, grams and Avogadro’s number — you can calculate the number of atoms in the sample.
TL DR
To calculate the number of atoms in a sample, divide its weight in grams by the amu atomic mass from the periodic table, then multiply the result by Avogadro’s number: 6.02 x 10^23.
Express the relationship of the three pieces of information you need to calculate the number of atoms in the sample in the form of an equation. Scientists express atomic weights in terms of grams per mole, so the resulting equation looks like this: atomic weight expressed in atomic mass units = grams/mole. In scientific notation, it would appear like this: u = g/mole.
Look up the sample’s atomic weight on a periodic table of the elements. For example, boron has an atomic weight of 10.811 atomic mass units which you could also express as 10.811 grams per mole of the element. Plugging that figure into the above equation would look like this: 10.811 = g/mole.
Things You’ll Need
Nuclear Properties And Stability
Atomic nuclei consist of protons and neutrons bound together by the residual strong force. Because protons are positively charged, they repel each other. Neutrons, which are electrically neutral, stabilize the nucleus in two ways. Their copresence pushes protons slightly apart, reducing the electrostatic repulsion between the protons, and they exert the attractive nuclear force on each other and on protons. For this reason, one or more neutrons are necessary for two or more protons to bind into a nucleus. As the number of protons increases, so does the ratio of neutrons to protons necessary to ensure a stable nucleus . For example, although the neutron:proton ratio of 3 is 1:2, the neutron:proton ratio of 23892U is greater than 3:2. A number of lighter elements have stable nuclides with the ratio 1:1 . The nuclide 4020Ca is observationally the heaviest stable nuclide with the same number of neutrons and protons. All stable nuclides heavier than calcium-40 contain more neutrons than protons.
Also Check: Theory Of Everything 2 Song
Redefinition Of The Si Base Units
The definition of the dalton was not affected by the 2019 redefinition of SI base units, that is, 1 Da in the SI is still 112 of the mass of a carbon-12 atom, a quantity that must be determined experimentally in terms of SI units. However, the definition of a mole was changed to be the amount of substance consisting of exactly 6.02214076×1023 entities and the definition of the kilogram was changed as well. As a consequence, the molar mass constant is no longer exactly 1 g/mol, meaning that the number of grams in the mass of one mole of any substance is no longer exactly equal to the number of daltons in its average molecular mass.
How Do You Use Weighted Average To Calculate Atomic Mass
To use weighted average, we must take into account the mass and percentage abundance of each isotope. Lets use the data in the following table to show how weighted average is used to calculate the atomic mass of oxygen.
Calculating atomic mass
Solution
To calculate the atomic mass of oxygen using the data in the above table, we must first
- multiply the mass of each isotope by its corresponding natural abundance . But, since the abundance is in %, you must also divide each abundance value by 100.
And second,
- Sum the result to get the atomic mass of the element
Thus,
Atomic mass of oxygen = 15.995 amu + 16.999 amu + 17.999 amu
= 15.956612 amu + 0.0067996 amu + 0.035998 amu
= 15.9994096 amu
= 16.00 amu
Note that the abundance in percent always add up to 100 %. Meaning if the previous question had left out the percentage abundance value for oxygen-18, you could have gotten it by subtracting the sum of the percentage abundance for oxygen-16 and oxygen-17 from 100% to get the percentage abundance for oxygen-18.
That is: 100% = 0.2% for oxygen-18
Generally, you can apply this approach to figure out missing percentage abundance when you know the percentage abundance values for all, but one isotope.
Read Also: Geometry Segment Addition Postulate Worksheet
Formula To Calculate Average Atomic Mass:
Average Atomic Mass= M1f1 + M2f2 + M3f3 + M3f3 + + Mnfn
Where,
- M stands for the atomic mass of the particular isotope of the given element.
- F stands for the natural abundance of the particular isotope of the given element.
Follow the below-mentioned steps one by one.
Average Atomic Mass= M1f1 + M2f2 + M3f3 + M3f3 + + Mnfn
Examples
Example 1.
Find the Average atomic mass of hydrogen. If Isotopes 1H and 2H has natural abundance is 99.984% and 0.0156% respectively.
Example 2.
Find the Average atomic mass of hydrogen. If Isotopes 12C and 13C has natural abundance is 99.93% and 1.07% respectively.
Example 3.
Find the Average atomic mass of Carbon. If Isotopes 35Cl and 37Cl has natural abundance is75.76% and 24.24% respectively.
Chemistry Help With Isotope
Element X has two naturally occurring Isotopes. The one isotope has a mass of 78.92 amu and a relative abundance of 50.69%. The second isotope has a relative abundance of 49.31% and a mass of 80.92 amu. Calculate the atomic mass of the element. Identify the element
To calculate the average atomic mass, we must take the % abundance of each isotope and multiply it by its corresponding mass.
+ + + …. = average atomic mass.
First, we take each percent abundance and convert it into decimal form.
50.69% —> 0.5069
Next, we multiply the decimal forms by the masses.
As in everything with chemistry, we must round to the correct number of significant digits/figures. When there are multiple operations, we can underline the significant figure we were supposed to round to at each step. But we wait to round at the end. Keep as many digits as possible.
In this case, when we follow PEMDAS, we have to multiply the parenthesis first. When multiplying, you look at the number of sig figs of each of the given numbers and the answer will contain the fewest number of sig figs. The numbers in the all contain 4 sig figs. **I have underlined and bolded the significant figure that we need to keep in mind for the final answer**
40.004548 + 39.901652
Then, for addition and subtraction, we are supposed to round to the fewest number of decimal places. Even though right now, it looks like there are 6 decimal places, I actually should not look past the underlined digits.
You May Like: Parallax Error Chemistry
Examples Of Values Expressed In Atomic Mass Units
- A hydrogen-1 atom has a mass of 1.007 u .
- A carbon-12 atom is defined as having a mass of 12 u.
- The largest known protein, titin, has a mass of 3 x 106 Da.
- AMU is used to differentiate between isotopes. An atom of U-235, for example, has a lower AMU than one of U-238, since they differ by the number of neutrons in the atom.
What If Elements Have More Than Two Isotopes
For example:
Oxygen has three naturally occurring isotopes 16O, 17O and 18O. The average atomic mass of oxygen is 15.9994 amu. Given, the atomic weight of 16O is 15.995 amu, 17O is 16.999 amu and 18O is 17.999 amu. Also, 17O has 0.037 percent in nature. What are the other isotopes percent abundances?
Firstly, we have the abundance of one isotope which is 0.0037. So, the abundances of the other two remaining isotopes is = 0.99963.
Let x be the unknown abundance of 16O and other isotope abundance of 18O be .
Now, modifying equation , we get
* + * + * = 15.9994
or 15.995x 17.999x = 15.9994
or x = 0.9976
So, we get the abundance of 16O is 0.9976 and the abundance of 18O is = 0.00203.
Hence, the percent abundance of three isotopes are given by
16O=0.9976*100=99.76%
Also Check: What Does Capital G Mean In Physics
What Is Atomic Mass
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. However, electrons have so much less mass than protons and neutrons that they don’t factor into the calculation. So, the atomic mass is the sum of the masses of protons and neutrons. There are three ways to find atomic mass, depending on your situation. Which one to use depends on whether you have a single atom, a natural sample of the element, or simply need to know the standard value.
Read A Brief Summary Of This Topic
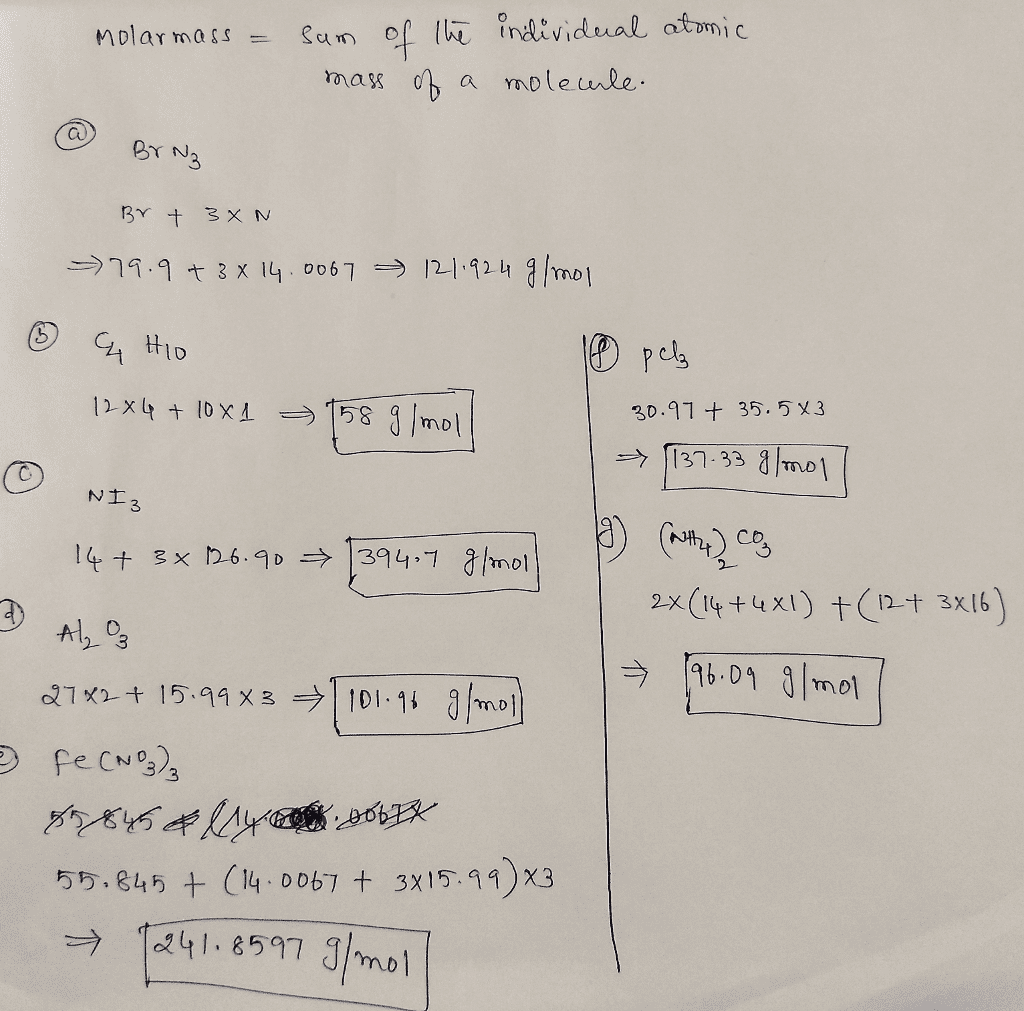
atomic weight, also called relative atomic mass, ratio of the average mass of a chemical elements atoms to some standard. Since 1961 the standard unit of atomic mass has been one-twelfth the mass of an atom of the isotopecarbon-12. An isotope is one of two or more species of atoms of the same chemical element that have different atomic mass numbers . The atomic weight of helium is 4.002602, the average that reflects the typical ratio of natural abundances of its isotopes. Atomic weight is measured in atomic mass units , also called daltons. See below for a list of chemical elements and their atomic weights.
The concept of atomic weight is fundamental to chemistry, because most chemical reactions take place in accordance with simple numerical relationships among atoms. Since it is almost always impossible to count the atoms involved directly, chemists measure reactants and products by weighing and reach their conclusions through calculations involving atomic weights. The quest to determine the atomic weights of elements occupied the greatest chemists of the 19th and early 20th centuries. Their careful experimental work became the key to chemical science and technology.
Recommended Reading: Holt Geometry Worksheet Answer Key
Use Of Nuclear Properties
- A technique similar to radioisotopic labeling is radiometric dating: using the known half-life of an unstable element, one can calculate the amount of time that has elapsed since a known concentration of isotope existed. The most widely known example is radiocarbon dating used to determine the age of carbonaceous materials.
- Several forms of spectroscopy rely on the unique nuclear properties of specific isotopes, both radioactive and stable. For example, nuclear magnetic resonance spectroscopy can be used only for isotopes with a nonzero nuclear spin. The most common nuclides used with NMR spectroscopy are 1H, 2D, 15N, 13C, and 31P.
- Mössbauer spectroscopy also relies on the nuclear transitions of specific isotopes, such as 57Fe.
- Radionuclides also have important uses. Nuclear power and nuclear weapons development require relatively large quantities of specific isotopes. Nuclear medicine and radiation oncology utilize radioisotopes respectively for medical diagnosis and treatment.
How You Calculate Atomic Mass Unit
- Step 1: Write out the chemical formula of the compound whose Atomic Mass you want to calculate. The chemical formula provides the number of atoms in one molecule of a compound . For example, the chemical formula of phosphate is H3O4P.
- Step 2: Look up the atomic mass for each individual element in the given compound on the Periodic Table. This number is commonly found just below the letter symbol for the element. For our example, the atomic mass of hydrogen is 1.008 amu per atom, oxygen is 16.00 amu per atom and phosphorous is 30.97 amu per atom.
- Step 3: Multiply the result for every atom in Step 2 by the number of atoms in one molecule of the compound. Phosphate contains three atoms of hydrogen, four atoms of oxygen and one atom of phosphorous in one molecule. So for phosphate the calculations would be as follows 1.008 amu/atom x 3 hydrogen atoms per molecule = 3.024 amu of hydrogen per molecule, 16.00 amu/atom x 4 oxygen atoms per molecule = 64.00 amu of oxygen per molecule and 30.97 amu/atom x 1 phosphorous atom per molecule = 30.97 amu of phosphorous per molecule.
- Step 4: Add the numbers calculated for each atom in Step 3 together to determine the total amu for one molecule of the compound. So for phosphate the atomic mass is 3.024 + 64.00 + 30.97 = 97.99 amu in one molecule of phosphate.
Also Check: 6 Major Branches Of Chemistry
Calculating Average Atomic Mass
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance .
Average atomic mass = f1M1 + f2M2 + + fnMnwhere f is the fraction representing the natural abundance of the isotope and M is the mass number of the isotope.
The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. When data are available regarding the natural abundance of various isotopes of an element, it is simple to calculate the average atomic mass.
- For helium, there is approximately one isotope of Helium-3 for every million isotopes of Helium-4 therefore, the average atomic mass is very close to 4 amu .
- Chlorine consists of two major isotopes, one with 18 neutrons and one with 20 neutrons . The atomic number of chlorine is 17 .
To calculate the average mass, first convert the percentages into fractions . Then, calculate the mass numbers. The chlorine isotope with 18 neutrons has an abundance of 0.7577 and a mass number of 35 amu. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.
Average atomic mass of chlorine = + = 35.48 amu
Another example is to calculate the atomic mass of boron , which has two isotopes: B-10 with 19.9% natural abundance, and B-11 with 80.1% abundance. Therefore,
Average atomic mass of boron = + = 10.80 amu
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
Origin Of The Concept
The interpretation of the law of definite proportions in terms of the atomic theory of matter implied that the masses of atoms of various elements had definite ratios that depended on the elements. While the actual masses were unknown, the relative masses could be deduced from that law. In 1803 John Dalton proposed to use the atomic mass of the lightest atom, that of hydrogen, as the natural unit of atomic mass. This was the basis of the atomic weight scale.
For technical reasons, in 1898, chemist Wilhelm Ostwald and others proposed to redefine the unit of atomic mass as 116 of the mass of an oxygen atom. That proposal was formally adopted by the International Committee on Atomic Weights in 1903. That was approximately the mass of one hydrogen atom, but oxygen was more amenable to experimental determination. This suggestion was made before the discovery of the existence of elemental isotopes, which occurred in 1912. The physicist Jean Perrin had adopted the same definition in 1909 during his experiments to determine the atomic masses and Avogadro’s constant. This definition remained unchanged until 1961. Perrin also defined the “mole” as an amount of a compound that contained as many molecules as 32 grams of oxygen . He called that number the Avogadro number in honor of physicist Amedeo Avogadro.
Recommended Reading: How Did Geography Affect Early Greek Civilization