The Influence Of Thermal Excitation
Since the motions of the atoms in a molecule are determined by quantum mechanics, “motion” must be defined in a quantum mechanical way. The overall quantum mechanical motions translation and rotation hardly change the geometry of the molecule. In addition to translation and rotation, a third type of motion is molecular vibration, which corresponds to internal motions of the atoms such as bond stretching and bond angle variation. The molecular vibrations are harmonic , and the atoms oscillate about their equilibrium positions, even at the absolute zero of temperature. At absolute zero all atoms are in their vibrational ground state and show zero point quantum mechanical motion, so that the wavefunction of a single vibrational mode is not a sharp peak, but an exponential of finite width . At higher temperatures the vibrational modes may be thermally excited , but they oscillate still around the recognizable geometry of the molecule.
To get a feeling for the probability that the vibration of molecule may be thermally excited,we inspect the Boltzmann factorβ â¡ exp, where ÎE is the excitation energy of the vibrational mode, k the Boltzmann constant and T the absolute temperature. At 298 K , typical values for the Boltzmann factor β are:
- β = 0.0890 for ÎE = 0500 cmâ1
- β = 0.0080 for ÎE = 1000 cmâ1
- β = 0.0007 for ÎE = 1500 cmâ1.
What Is Molecular Geometry
Molecular geometry is the shape of a molecule predicted by considering only bond electron pairs. In this case, lone electron pairs are not taken into account. Moreover, double bonds and triple bonds are considered as single bonds. The geometries are determined based on the fact that lone electron pairs need more space than bonding electron pairs. For example, if a certain molecule is composed of two pairs of bonding electrons along with a lone pair, the molecular geometry is not linear. The geometry there is bent or angular because the lone electron pair needs more space than two bonding electron pair.
Chapter 6 Molecular Structure Of Compounds
- Predict the structures of small molecules using valence shell electron pair repulsion theory
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space . A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are measured in Ångstroms or picometers .
Figure 1. Bond distances and angles are shown for the formaldehyde molecule, H2CO.
Also Check: New England Geography And Climate
What Is The Electron Domain Geometry And Molecular Geometry For
According to VSEPR, electron pairs distribute themselves around a central atom in such a way as to maximize their distance from each other. Electron domain geometries are based on the total number of electron pairs, while molecular geometries describe the arrangement of atoms and bonding pairs in a molecule.
Types Of Molecular Structure
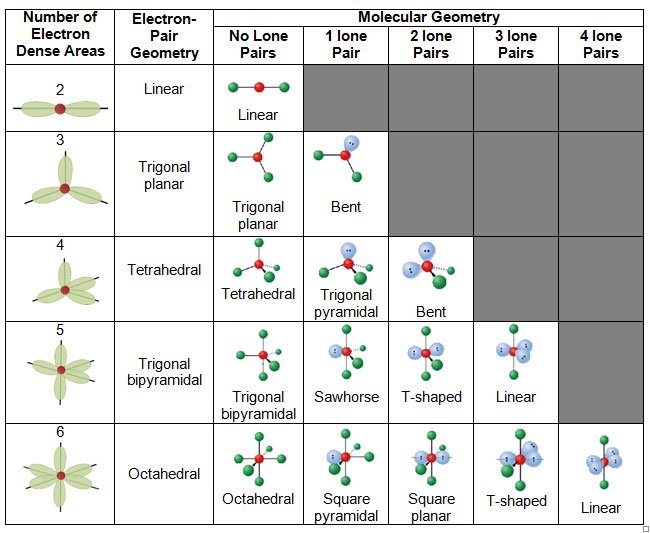
A bond angle is the geometric angle between two adjacent bonds. Some common shapes of simple molecules include:
The bond angles in the table below are ideal angles from the simple VSEPR theory , followed by the actual angle for the example given in the following column where this differs. For many cases, such as trigonal pyramidal and bent, the actual angle for the example differs from the ideal angle, and examples differ by different amounts. For example, the angle in H2S differs from the tetrahedral angle by much more than the angle for H2O does.
| Atoms bonded to |
|---|
Read Also: Mcgraw Hill Geometry Answers
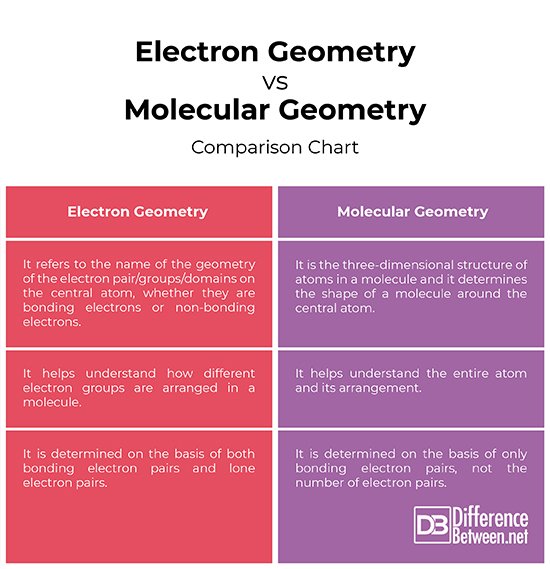
Electron Geometry Vs Molecular Geometry
Geometry in chemistry refers to the shape of molecules in 3-Dimensional space.
Molecular geometry is described as the 3D arrangement of atoms in a molecule, normally relative to a single central atom. Whereas, electron geometry is the 3D arrangement of electron pairs around a central atom, whether bonding or non-bonding.
A lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond. And a bond pair is a pair of electrons in a bond.
It is well known that the electron pairs, being negatively charged, repel each other. This repulsion causes the electron pairs around the central atom to arrange as far apart from each other as possible. This minimizes the repulsion.
Under the influence of a single nucleus, a lone pair offers more repulsion than a bond pair which is influenced by two nuclei. This causes a slight decrease in bond angles .
If all of the electron groups are bond pairs , the molecular geometry and electron geometry are the same. An example is a methane molecule, CH4 with 4 bond pairs and no lone pairs, all 4 of carbons valence electrons are bonded with hydrogen atoms. Its molecular, as well as electronic geometry, is tetrahedral.
Prerequisite concept:
- Lewis Structure
- Valence Shell Electron Pair Repulsion Theory
Solution For Problem 69p Chapter 10
Introductory Chemistry | 5th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Introductory Chemistry | 5th Edition
Determine the molecular geometry of each molecule.
Step 1 of 5
Here, we are going to find out electron and molecular geometries of each molecule.The bond angles can be calculated by using VSEPR theory. The repulsion between the electron groups of the internal atom determines the geometry of the molecule. The best possible geometry of the molecule is that where electron groups have maximum separation between them. The VSEPR notation for a molecule is as follows
AXn, where “A” and n represents central atom and the number of bonds with the central atom respectively.
When lone pair of electrons are present in a molecule, then the VSEPR notation will be AXnEn, where En is the number of lone pair of electrons present on the central atom.,
Electron group geometry depends on the number of electron groups around the central atom.
Molecular geometry of the molecule can be determined by electron groups as well lone pair of electrons.
___________________________________
Recommended Reading: Movement Geography Example
What Is The Difference Between Electron Geometry And Molecule Geometry
Considering the definition of electron geometry and molecule geometry, the main difference between electronic geometry and molecular geometry is that electronic geometry includes the bonds and pairs of electrons in the molecule, whereas molecular geometry includes only the number of bonds that are present in a molecule.
- Molecular geometry
Molecular geometry refers to the three-dimensional arrangement of the atoms that make up a molecule. It is a determinant of many of the compound’s properties, for example, boiling point, density, or solubility.
Molecular geometry can be predicted using the Valencia Shell Electronic Pair Repulsion Theory, which is based on the fact that electrons tend to repel each other . For this reason, the orbitals that contain the electrons are oriented in such a way that they are as far away from each other.
- Electronic geometry
On the other hand, electronic geometry is the way in which electrons are arranged in space, considering all the valence pairs of the central atom. Generally matches molecular geometry but not always.
- Difference
So, the electron geometry depends on the structure of the electrons of the central atom of a molecule, while the molecular geometry depends on whether other atoms are attached to the central atom or if there are pairs of free electrons.
Learn more:
When Is Molecular Geometry Different From Electron Geometry
Asked by: Mr. Laverna Smitham PhD
Re: Difference between molecular and electron geometry? Electron geometry describes the arrangement of electron groups. Molecular geometry describes the arrangement of atoms, excluding lone pairs. For example, in the case of a trigonal planar shape as defined by electron geometry, there are three bonds.
Also Check: Kmt Definition Chemistry
Trigonal Bipyramidal Electron Geometry
Trigonal bipyramidal is a central atom with five pairs of bonding electron pairs.
The geometrical name comes from the shape of three pairs in a plane at 120-degree angles and the remaining two pairs at 90-degree angles to the plane. The shape resembles two pyramids with a triangular base attached together.
It is important to note that the lone electron pairs will fill the trigonal planar portion of the geometry first. Only the trigonal bipyramidal shape has the same electron geometry and molecular geometry:
- trigonal bipyramidal: all five pairs of bonding electrons are bonded to atoms
- seesaw: four atoms bonded, one lone pair of electrons
- t-shaped: three atoms bonded, two lone pairs of electrons
- linear: two atoms bonded , three lone pairs of electrons
Difference Between Electron Pair Geometry And Molecular Geometry
Electron Pair Geometry vs Molecular Geometry
The geometry of a molecule is important in determining its properties like color, magnetism, reactivity, polarity, etc. There are various methods of determining the geometry. There are many types of geometries. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, octahedral are some of the commonly seen geometries.
What is Molecular Geometry?
Molecular geometry is the three dimensional arrangement of atoms of a molecule in the space. Atoms are arranged in this way, to minimize the bond-bond repulsion, bond-lone pair repulsion and lone pair-lone pair repulsion. Molecules with the same number of atoms and electron lone pairs tend to accommodate the same geometry. Therefore, we can determine the geometry of a molecule by considering some rules. VSEPR theory is a model, which can be used to predict the molecular geometry of molecules, using the number of valence electron pairs. However, if the molecular geometry is determined by the VSEPR method, only the bonds should be taken into consideration, not the lone pairs. Experimentally the molecular geometry can be observed using various spectroscopic methods and diffraction methods.
What is Electron Pair Geometry?
Atoms in a molecule are bound together by electron pairs. These are called bonding pairs.
Some atoms in a molecule may also possess pairs of electron not involved in bonding. These are called lone pairs.
Lone pairs occupy more space than bonding pairs.
Also Check: Lewis Structure For F2o
Lewis Structures With Wedge
The Lewis structure gives us meaningful information about the bonds between atoms, but Lewis structures do not depict how the molecule exists in three-dimensions. Depicted on the left in Figure 1 is the Lewis structure for methane showing the central carbon atom singly bonded to four hydrogen atoms:
Figure 1.
The Lewis structure is misleading because it depicts methane as a planar plus-shaped molecule, yet we know that methane is a non-planar, tetrahedral molecule. Representing three dimensions on 2D paper is challenging. Chemists use dash and wedge notation to draw 3D molecules. Bonds drawn as simple lines are located in the plane of the page or screen. Bonds drawn with a solid triangle, or wedge, represent bonded atoms coming out of the page/screen towards the observer bonds drawn with dashed triangle, or dash, represent bonded atoms going into the page/screen away from the observer. The correct Lewis structure with dash and wedge notation for CH4 is depicted on the right in Figure 1. It communicates the structure of methane more clearly than a traditional Lewis structure without dash and wedge notation
The dash and wedge notation is shown again below adjacent to a space-filling model of CH4 and the ball and stickmodel . All three images represent the same bond placements in space .
Figure 2.
Example : Predicting Electron
Predict the electron-pair geometry and molecular structure for phosgene, COCl2, a chemical warfare agent used during World War I
This shows us three regions of high electron density around the carbon atomeach single bond counts as one region, and the double bond counts as another region, and there are no lone pairs on the carbon atom. Using VSEPR theory, we predict that the three regions of electron density arrange themselves around the central atom with a bond angle of 120°. The electron-pair geometry and molecular structure are identical because all the groups are bonding, and COCl2 molecules are trigonal planar.
The next several examples illustrate the effect of lone pairs of electrons on molecular structure.
Also Check: Algebra Age Word Problems
Electron Geometry Vs Molecular Geometry: Whats The Difference
Interesting and intriguing chemistry is always fun! While understanding what matter is made of, we learn about so many new things that we simply lose ourselves in the beautiful world of chemistry.
However, a few concepts can be slightly difficult to comprehend because they seem similar or because they are just confusing! One such concept is the difference between electron geometry and molecular geometry. To help you out, we are shedding light on electron geometry vs molecular geometry in this article.
Electron geometry teaches us about the arrangement of different electron groups. Molecular geometry, on the other hand, helps us understand the entire atom and its arrangement. It is the 3D arrangement of all the atoms in a particular molecule.
So, when you compare them, you will note that atoms have different arrangements in electron geometry and molecular geometry.
Determination Of Molecular Geometry
The shape of a molecule is determined by the bonded atom, although this does not mean the shape itself is unaffected by the presence of lone pair.
Molecular geometry includes geometrical parameters such as bond lengths, bond angles, and torsional angles that help determine the position of atoms as well as a molecules general shape.
It influences a substances properties such as its reactivity, color, polarity, magnetism, biological activity, and phase of matter.
There are various techniques to determine molecular geometry such as Raman spectroscopy, infrared spectroscopy, and microwave spectroscopy.
Don’t Miss: Beth Thomas Now
S Used To Find The Shape Of The Molecule
To sum up there are four simple steps to apply the VSEPR theory.
Main Difference Electron Geometry Vs Molecular Geometry
The geometry of a molecule determines the reactivity, polarity and biological activity of that molecule. The geometry of a molecule can be given as either the electron geometry or the molecular geometry. The VSEPR theory can be used to determine the geometries of molecules. Electron geometry includes the lone electron pairs present in a molecule. Molecular geometry can be determined by the number of bonds that a particular molecule has. The main difference between electron geometry and molecular geometry is that electron geometry is found by taking both lone electron pairs and bonds in a molecule whereas molecular geometry is found using only the bonds present in the molecule.
Don’t Miss: Eoc Fsa Practice Test Algebra 1
Determination Of Electron Geometry
Since electrons are always moving and their paths cannot be accurately figured, the arrangement of electrons is described in terms of electron density distribution.
Electron geometry is determined by the number of electron pairs. The following table gives an idea of electronic geometry according to the number of electron pairs.
What Is Electron Geometry
Electron geometry is the shape of a molecule predicted by considering both bond electron pairs and lone electron pairs. The VSEPR theory states that electron pairs located around a certain atom repel each other. These electron pairs can be either bonding electrons or non-bonding electrons.
The electron geometry gives the spatial arrangement of all the bonds and lone pairs of a molecule. The electron geometry can be obtained using VSEPR theory.
Don’t Miss: How To Calculate Displacement Physics
Molecules With More Than One Central Atom
The VSEPR theory not only applies to one central atom, but it applies to molecules with more than one central atom. We take in account the geometric distribution of the terminal atoms around each central atom. For the final description, we combine the separate description of each atom. In other words, we take long chain molecules and break it down into pieces. Each piece will form a particular shape. Follow the example provided below:
Butane is C4H10. C-C-C-C is the simplified structural formula where the Hydrogens are implied to have single bonds to Carbon. You can view a better structural formula of butane at en.Wikipedia.org/wiki/File:Butane-2D-flat.png If we break down each Carbon, the central atoms, into pieces, we can determine the relative shape of each section. Let’s start with the leftmost side. We see that C has three single bonds to 2 Hydrogens and one single bond to Carbon. That means that we have 4 electron groups. By checking the geometry of molecules chart above, we have a tetrahedral shape. Now, we move on to the next Carbon. This Carbon has 2 single bonds to 2 Carbons and 2 single bonds to 2 Hydrogens. Again, we have 4 electron groups which result in a tetrahedral. Continuing this trend, we have another tetrahedral with single bonds attached to Hydrogen and Carbon atoms. As for the rightmost Carbon, we also have a tetrahedral where Carbon binds with one Carbon and 3 Hydrogens.
Predicting Electron Pair Geometry And Molecular Structure
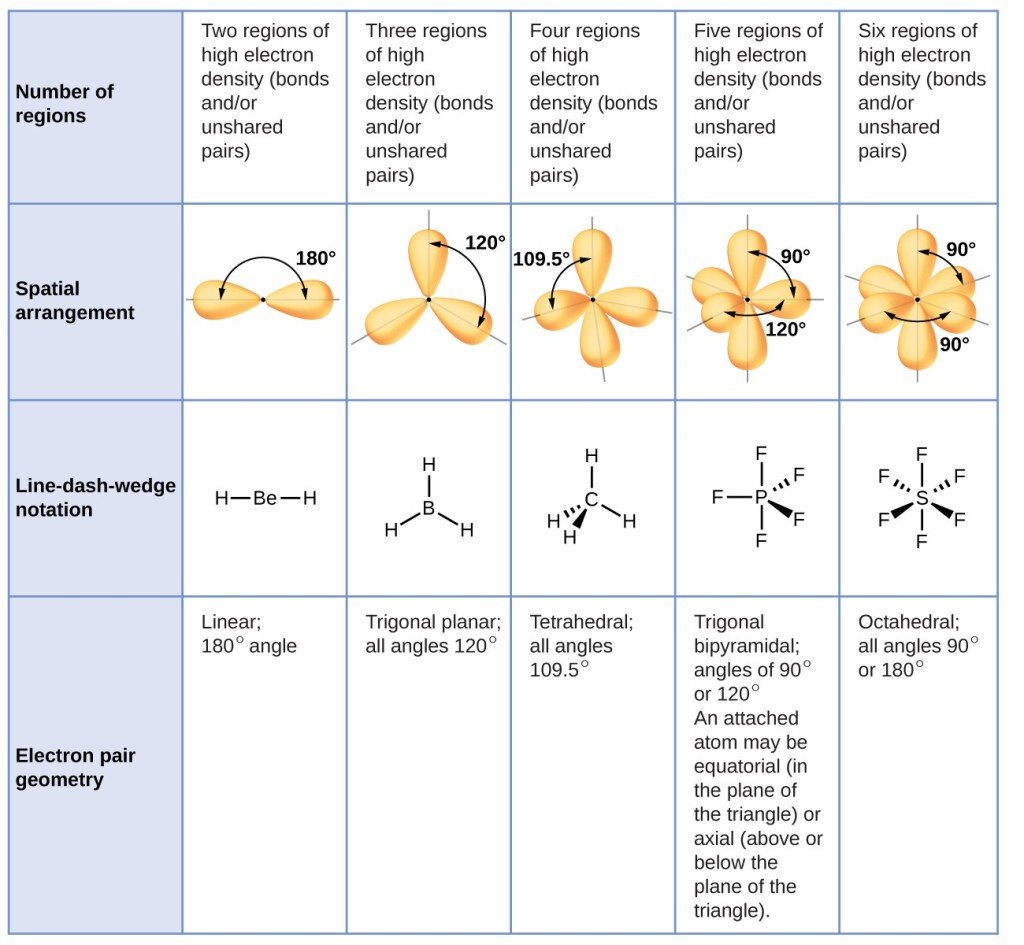
The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures:
The following examples illustrate the use of VSEPR theory to predict the molecular structure of molecules or ions that have no lone pairs of electrons. In this case, the molecular structure is identical to the electron pair geometry.
You May Like: What Is Span Linear Algebra